Your Zero order reaction example images are available. Zero order reaction example are a topic that is being searched for and liked by netizens now. You can Download the Zero order reaction example files here. Get all royalty-free photos.
If you’re looking for zero order reaction example pictures information linked to the zero order reaction example topic, you have visit the right blog. Our website frequently provides you with suggestions for downloading the highest quality video and image content, please kindly surf and find more informative video articles and images that fit your interests.
Zero Order Reaction Example. R a t e k H 2 0 C l 2 0. A rightarrow P is a reaction that occurs in zero order. I If the rate constant of a reaction is k 3 10-4 s-1 then identify the order of the reaction. 2N 2 O g 2N 2g O 2g The rate of this reaction is equal to the rate constant.
 Define Zero Order Reaction With Example Chemistry Chemical Kinetics 12752715 Meritnation Com From meritnation.com
Define Zero Order Reaction With Example Chemistry Chemical Kinetics 12752715 Meritnation Com From meritnation.com
T 12 is the half-life. One example of this type of correlation is the Pearson. R a t e k H 2 0 C l 2 0. R a t e k. And this reaction occurs on the surface of a metal catalyst. Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction.
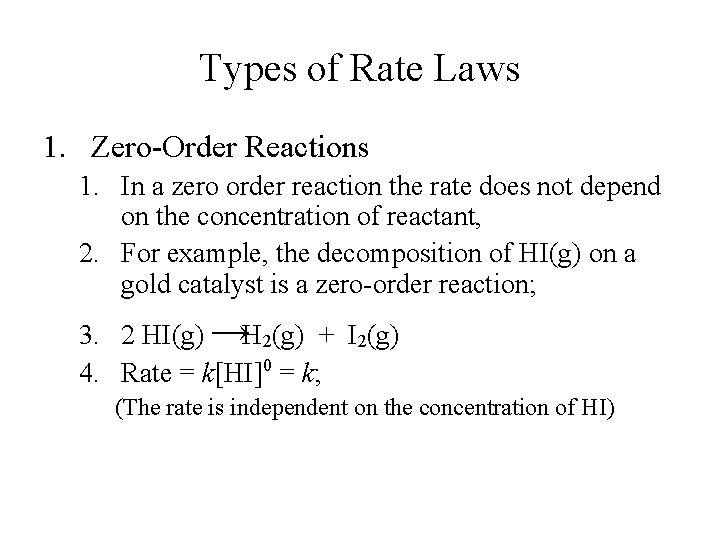
The order of the reaction can be defined as the sum of powers of the concentration of the reactants in the rate law expression.
In this type of reaction the limiting factor is something other than concentration for example solubility or absorption of light in certain photochemical reactions. In this type of reaction the limiting factor is something other than concentration for example solubility or absorption of light in certain photochemical reactions. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line. 1 Kinetic order elimination equation where delta drug represents the change in plasma concentration of the drug divided by time n represents either first or zero-order elimination with 1 or 0 respectively and -Kc represents a constant. So in this sense zero-order reactions have to have a more complex nature such as involving an enzyme as in alcohol decomposition. The rate of reaction is studied by nothing the rate at which water rises in the vessel due to dissociation of HCl formed.
 Source: youtube.com
Source: youtube.com
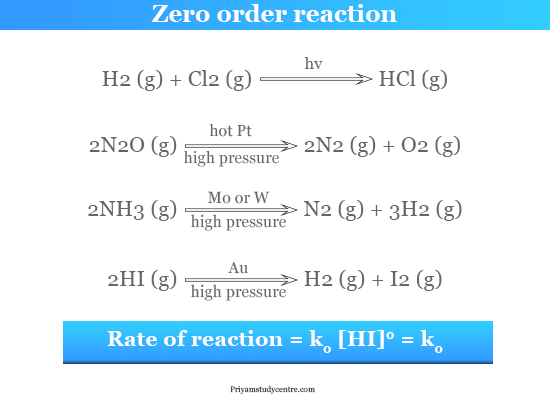
One example of this type of correlation is the Pearson. In these reactions there may be multiple reactants present but only one reactant will be of first-order concentration while the rest of the reactants would be of zero-order concentration. The reaction of hydrogen with chlorine is known as a photochemical reaction which is a zero-order reaction. The order of a reaction is simply the sum of the exponents on the concentration terms for a rate law. Rate kN 2 O g 0.
 Source: slideplayer.com
Source: slideplayer.com
A rightarrow P is a reaction that occurs in zero order. Rate k A3B05 is 3rd order in A half order in B and 35 order overall. In these reactions there may be multiple reactants present but only one reactant will be of first-order concentration while the rest of the reactants would be of zero-order concentration. We want to construct a stability diagram. Bimolecular reactions such as atommolecule moleculemolecule reactions are very common and strictly involve only two species.
 Source: youtube.com
Source: youtube.com
Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction. Both zero and first-order kinetics derive from the same equation. Rate k A1B0 k A is 1st order in A and 0th order in B and 1st order for the reaction. One of the most basic types of correlation is known as zero-order correlation which refers to the correlation between two variables without controlling for the possible influence of other variables. H 2 C l 2 h v 2 H C l.

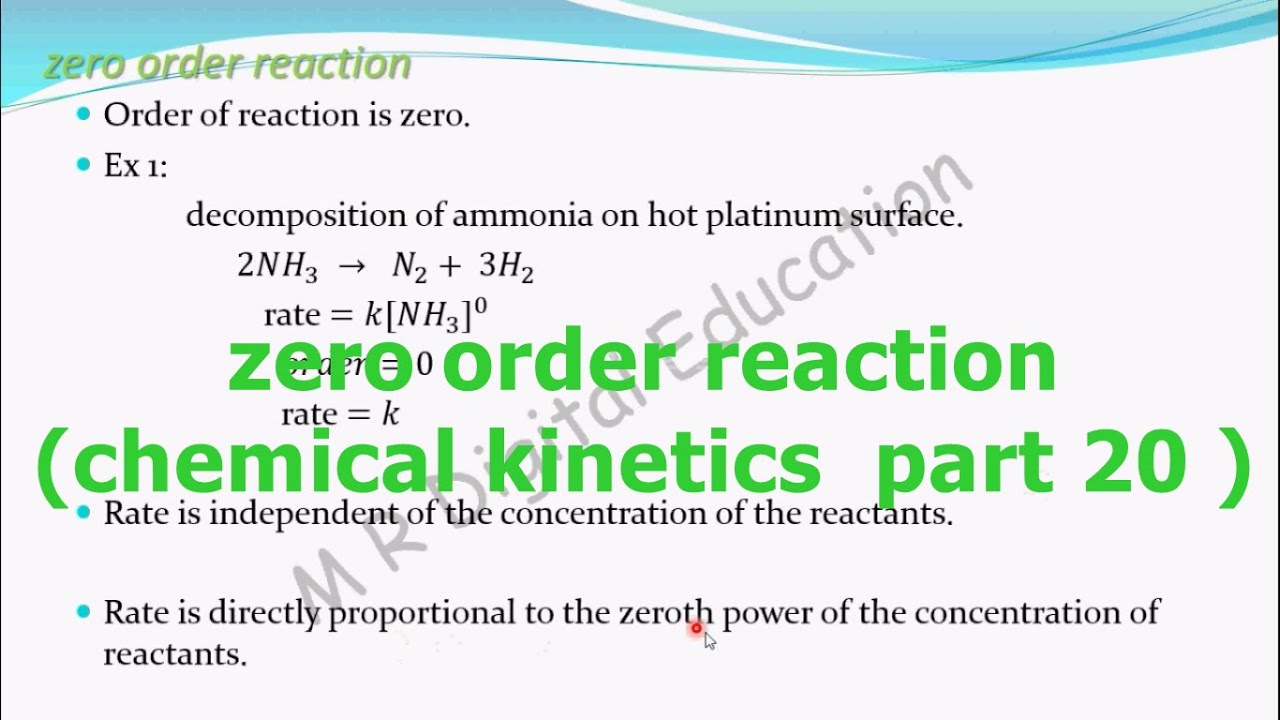
The rate constant for the reaction can be determined from the slope of the line which is equal to -k. So lets say that were using platinum here. 2H 2 O 2 2H 2 O O 2. So the decomposition of ammonia into nitrogen and hydrogen. H 2 C l 2 h v 2 H C l.
 Source: slideplayer.com
Source: slideplayer.com
Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction. For example the rate law for the reaction. Rate k A1B0 k A is 1st order in A and 0th order in B and 1st order for the reaction. H 2 C l 2 h v 2 H C l. For more video Boyles law httpsyoutubel2h1-sGI00kJEE main 2021 question from chemical kinetics httpsyoutubeq2Nl3VeeojwCurtius reaction and thei.

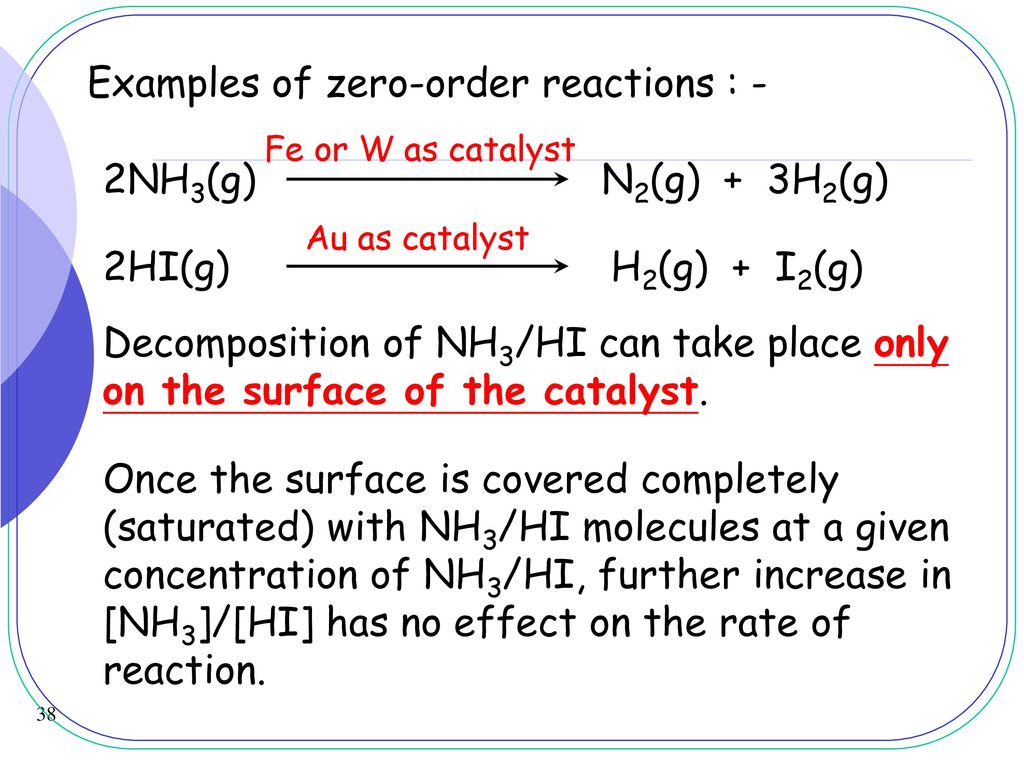
Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction. The rate of rise of water is the same. The order of a reaction is simply the sum of the exponents on the concentration terms for a rate law. Bimolecular reactions such as atommolecule moleculemolecule reactions are very common and strictly involve only two species. Decomposition of nitrous oxide over a hot plate of platinum acting as a catalyst surface.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
H 2 g C l 2 g h ν 2 H C l g 2. Bimolecular reactions such as atommolecule moleculemolecule reactions are very common and strictly involve only two species. The rate constant for the reaction can be determined from the slope of the line which is equal to -k. The rate law for a zero order reaction is A A0 - kt. Construct the stability curves for a zero order reaction S 0 and a first order reaction S 1 as a function of T C.

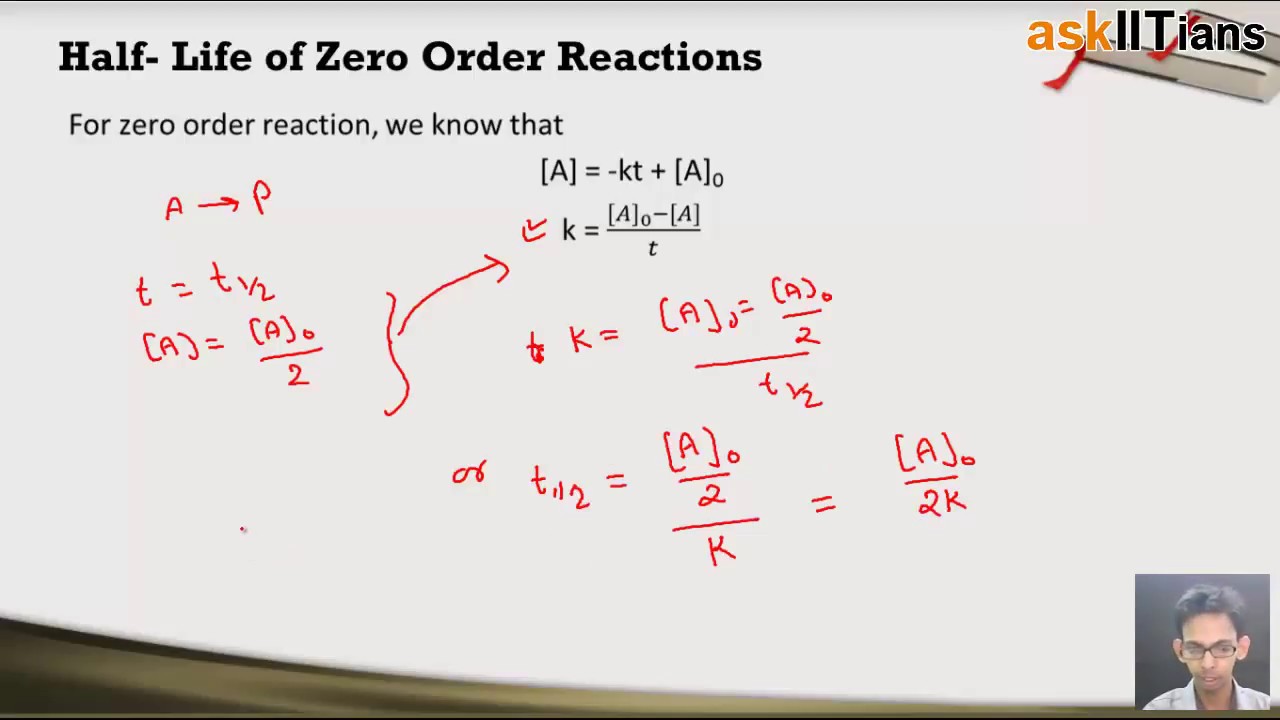
Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction. To find the half-life for a zero order reaction the equation t12 A0 2k is used. Lets look at an example of a zero order reaction and this will help us understand this idea of half life a little bit better. R a t e k. So our example is the decomposition of ammonia.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
H 2 g Cl 2 g 2HClg. The reaction of hydrogen with chlorine is known as a photochemical reaction which is a zero-order reaction. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line. K is the temperature-dependent reaction rate constant. The graph of reactant concentration vs.
 Source: khanacademy.org
Source: khanacademy.org
One of the most basic types of correlation is known as zero-order correlation which refers to the correlation between two variables without controlling for the possible influence of other variables. The integrated rate law for the zero-order reaction A products is A_t -kt A_0. And this reaction occurs on the surface of a metal catalyst. One of the most basic types of correlation is known as zero-order correlation which refers to the correlation between two variables without controlling for the possible influence of other variables. For example the rate law for the reaction.
 Source: youtube.com
Source: youtube.com
K is the temperature-dependent reaction rate constant. The rate constant k of an -order reaction has dimensions. And this reaction occurs on the surface of a metal catalyst. Where is called the order of the reaction in A is called the order of the reaction in B and the sum of the exponents is called the order of the reaction. In this type of reaction the limiting factor is something other than concentration for example solubility or absorption of light in certain photochemical reactions.
 Source: chemistrylearner.com
Source: chemistrylearner.com
To find the half-life for a zero order reaction the equation t12 A0 2k is used. For example the rate law for the reaction. The rate constant k of an -order reaction has dimensions. 1492 d2ϕ dY2 ϑ 2ϕ 0 0 Y δδS δ. And this reaction occurs on the surface of a metal catalyst.
 Source: toppr.com
Source: toppr.com
Decomposition of nitrous oxide over a hot plate of platinum acting as a catalyst surface. T 12 is the half-life. What percentage of A concentration is left after 297 seconds if the initial. So in this sense zero-order reactions have to have a more complex nature such as involving an enzyme as in alcohol decomposition. Note also that the order of a reaction is measured experimentally as the sum of the.
 Source: meritnation.com
Source: meritnation.com
H 2 C l 2 h v 2 H C l. The reaction is studied by placing H 2 and Cl 2 gases over water. A 0 is the initial concentration. Rate k A1B0 k A is 1st order in A and 0th order in B and 1st order for the reaction. Thus the concentration changes linearly with time.
 Source: slidetodoc.com
Source: slidetodoc.com
In these reactions there may be multiple reactants present but only one reactant will be of first-order concentration while the rest of the reactants would be of zero-order concentration. The order of the reaction can be defined as the sum of powers of the concentration of the reactants in the rate law expression. The rate law for a zero order reaction is A A0 - kt. One of the most basic types of correlation is known as zero-order correlation which refers to the correlation between two variables without controlling for the possible influence of other variables. In these reactions there may be multiple reactants present but only one reactant will be of first-order concentration while the rest of the reactants would be of zero-order concentration.
 Source: byjus.com
Source: byjus.com
The zero-order reaction as a limiting case of MichaelisMenten kinetics can be important during biochemical reactions therefore this case is briefly discussed in this section. H 2 g C l 2 g h ν 2 H C l g 2. To find the half-life for a zero order reaction the equation t12 A0 2k is used. Bimolecular reactions such as atommolecule moleculemolecule reactions are very common and strictly involve only two species. As seen in Equation No.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
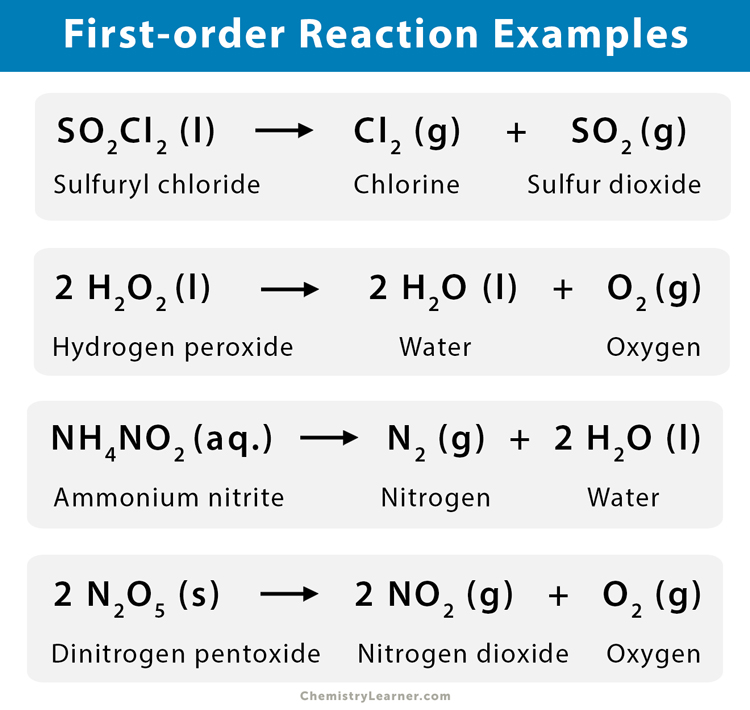
The reaction of hydrogen with chlorine is known as a photochemical reaction which is a zero-order reaction. Examples of Zero Order Reactions. Examples of Zero Order Reactions. Photochemical reaction between hydrogen and chlorine. The graph of reactant concentration vs.
 Source: chegg.com
Source: chegg.com
Photochemical reaction between hydrogen and chlorine. Construct the stability curves for a zero order reaction S 0 and a first order reaction S 1 as a function of T C. Bimolecular reactions such as atommolecule moleculemolecule reactions are very common and strictly involve only two species. The reaction is studied by placing H 2 and Cl 2 gases over water. 1 Kinetic order elimination equation where delta drug represents the change in plasma concentration of the drug divided by time n represents either first or zero-order elimination with 1 or 0 respectively and -Kc represents a constant.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title zero order reaction example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






