Your Violation of hunds rule example images are ready. Violation of hunds rule example are a topic that is being searched for and liked by netizens today. You can Download the Violation of hunds rule example files here. Download all royalty-free photos and vectors.
If you’re searching for violation of hunds rule example images information related to the violation of hunds rule example keyword, you have come to the right site. Our website always gives you hints for seeking the highest quality video and picture content, please kindly surf and find more enlightening video content and images that fit your interests.
Violation Of Hunds Rule Example. So the answer is b. There must be one electron with the same spin in each orbital of the same energy before you can put two in the same orbital. What is Hunds rule of maximum multiplicity explain with example. Use Hunds rule to derive the electronic configuration of Ce3 ion and calculate its magnetic moment on the basis of spin-only formula.
 Difference Between Pauli Exclusion Principle And Hund Rule From newsstellar.com
Difference Between Pauli Exclusion Principle And Hund Rule From newsstellar.com
How many electrons can be described by the set of quantum numbers n 4 l 2ml 0 ms ½. You have two electrons in one 2p orbital but none in the other 2p orbitals. Recognizing examples and non-examples of Hunds Rule. Is the Pauli Exclusion Principle ever violated. The maximum number of electrons that can occupy an orbital labeled dxy is A 1 B 2 C 3 D 4 Q. Which of the following orbital diagrams is incorrect because it violates hunds rule.
Hunds rule says that the highest value of 2S1 conforms to the lowest energy or the most stable configuration.
Hunds rule says that the highest value of 2S1 conforms to the lowest energy or the most stable configuration. According to Hunds rule the two 2s electrons will occupy the same orbital but the two 2p electrons will occupy distinct orbitals. A violates Hunds Rule since electrons in 2p are doubled up before each of the orbitals at that energy have one in them b violates the Aufbau principle because the 1s orbital is missing an electron. Ignoring the Hund principle the Aufbau principle states that electrons have to first fill the lowest energy levels. What is Hunds rule give example. How many electrons can be described by the set of quantum numbers n 4 l 2ml 0 ms ½.
 Source: vedantu.com
Source: vedantu.com
Now the energy level of the s-orbital has to be the lowest and only in the option b the s-orbital is not filled fully. Electron Configuration and its Purpose The electrical configuration of an atom is the distribution of electrons into orbitals. Same energy orbitals are available one electron goes into each until all of them are half full before pairing up. According to Hunds rule all orbitals will be singly occupied before any is doubly occupied. This violates Hunds Rule.
 Source: clutchprep.com
Source: clutchprep.com
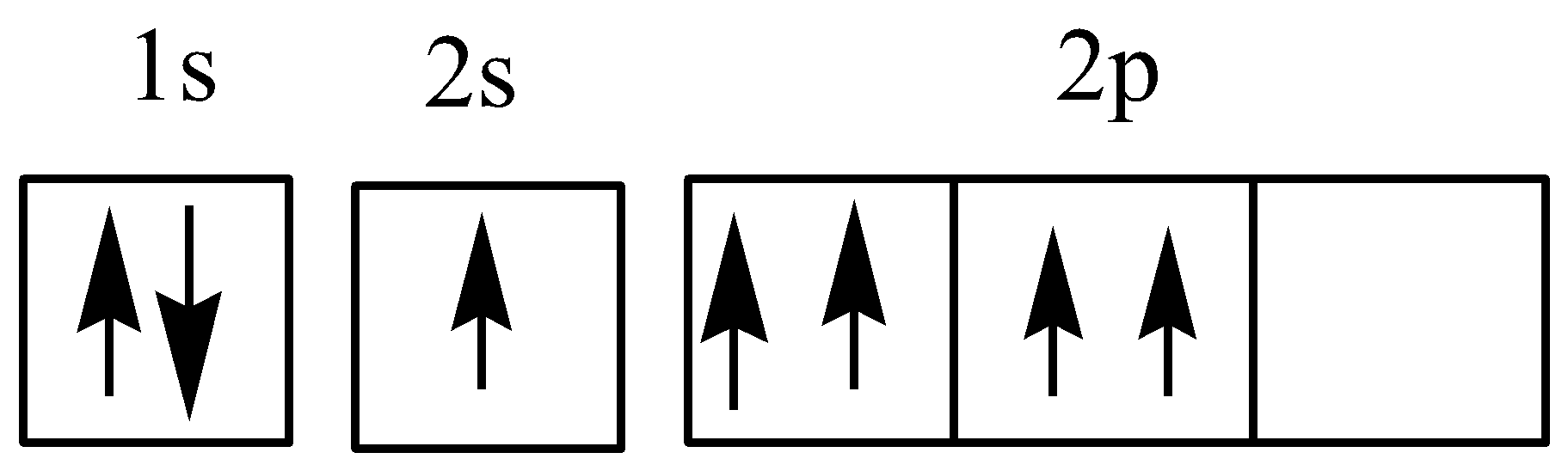
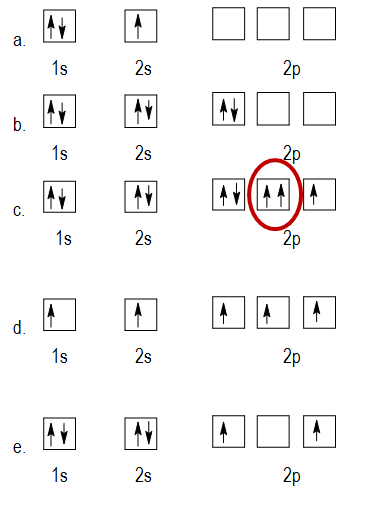
Similarly in the second figure 2Pz orbital is empty while 2P x and 2P y orbital are filled with two electrons. Therefore two p orbital get one electron and one will have two electrons. The image attached is the example of hunds rule. Which electron configuration represents a violation of Hunds Rule. You have two electrons in one 2p orbital but none in the other 2p orbitals.
 Source: numerade.com
Source: numerade.com
Violation of Hunds rule examples In the first figure the 2P x orbital contains two electrons before 2P y and 2P z orbitals are filled singly. Is the Pauli Exclusion Principle ever violated. What is Hunds rule give example. Thus it can be said that if Hunds rule violated only one unpaired electrons is present in left Crleft NH_3 right_6 right3. Which electron configuration represents a violation of hunds rule for an atom in its ground state.
 Source: youtube.com
Source: youtube.com
Let us take the example of the 2p subshell. The image attached is the example of hunds rule. What is Aufbau principle and. A10 b5 c4 d1 e0. Recognizing examples and non-examples of Hunds Rule.
 Source: mcqpoint.com
Source: mcqpoint.com
The image attached is the example of hunds rule. This violates Hunds Rule. Therefore two p orbital get one electron and one will have two electrons. Use Hunds rule to derive the electronic configuration of Ce3 ion and calculate its magnetic moment on the basis of spin-only formula. Which electron configuration represents a violation of hunds rule for an atom in its ground state.
 Source: instasolv.com
Source: instasolv.com
How many electrons can be described by the set of quantum numbers n 4 l 2ml 0 ms ½. Same energy orbitals are available one electron goes into each until all of them are half full before pairing up. What is Hunds rule of maximum multiplicity explain with example. Recognizing examples and non-examples of Hunds Rule - YouTube. This violates Hunds Rule.
 Source: mysciencesquad.weebly.com
Source: mysciencesquad.weebly.com
Hunds rule is sometimes violated because the orbitals the electrons should be filling are more energetic than other configurations due to effects caused by quantum mechanics and general relativity. Properly fill in the orbitals of an atom that possesses 8 electrons within its d set of orbitals. Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied and all electrons in singly occupied orbitals have the same spin. Hunds Rule states that if 2 or more degenerate ie. This violates Hunds Rule.
 Source: clutchprep.com
Source: clutchprep.com
You have two electrons in one 2p orbital but none in the other 2p orbitals. Which electron configuration represents a violation of hunds rule for an atom in its ground state. Therefore two p orbital get one electron and one will have two electrons. According to Hunds rule the two 2s electrons will occupy the same orbital but the two 2p electrons will occupy distinct orbitals. What is Hunds rule give example.
 Source: newsstellar.com
Source: newsstellar.com
You have two electrons in one 2p orbital but none in the other 2p orbitals. The electron configuration of a carbon atom for example is 1s 2 2s 2 2p 2. Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied and all electrons in singly occupied orbitals have the same spin. The Pauli Exclusion Principle states that no two electrons can be identified by the same set of quantum numbers. Therefore two p orbital get one electron and one will have two electrons.
 Source: chemistnate.com
Source: chemistnate.com
For example for boron through neon the electron filling order of the 2p orbitals follows Hunds Rule. The maximum value is attained only in the case of identical spins. How many electrons can be described by the set of quantum numbers n 4 l 2ml 0 ms ½. Let us take the example of the 2p subshell. Paulis exclusion principle According to this law an orbital cannot have both the electrons in the same spin motion half-integer spin.
 Source: byjus.com
Source: byjus.com
The major difference between a 1s orbital and a 2s orbital is that. Mar 2 2009 6 persundqvist 111 0. Use Hunds rule to derive the electronic configuration of Ce3 ion and calculate its magnetic moment on the basis of spin-only formula. The maximum number of electrons that can occupy an orbital labeled dxy is A 1 B 2 C 3 D 4 Q. The electron configuration of a carbon atom for example is 1s 2 2s 2 2p 2.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
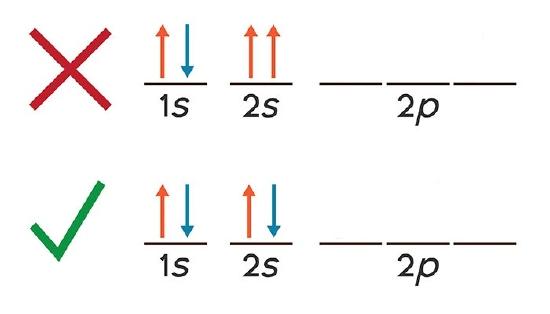
Thus it can be said that if Hunds rule violated only one unpaired electrons is present in left Crleft NH_3 right_6 right3. In which of the following diagrams is the Aufbau principle violated. Therefore two p orbital get one electron and one will have two electrons. Electrons will be in either positive half spin 12 or negative half spin -12 For example argons electron configuration. Similarly in the second figure 2Pz orbital is empty while 2P x and 2P y orbital are filled with two electrons.
 Source: clutchprep.com
Source: clutchprep.com
What is Hunds rule explain with example. What is Hunds rule of maximum multiplicity explain with example. This violates Hunds Rule. Similarly in the second figure 2Pz orbital is empty while 2P x and 2P y orbital are filled with two electrons. Question 4 The electrons in the half-filled 4d orbitals dont all have the same spin.
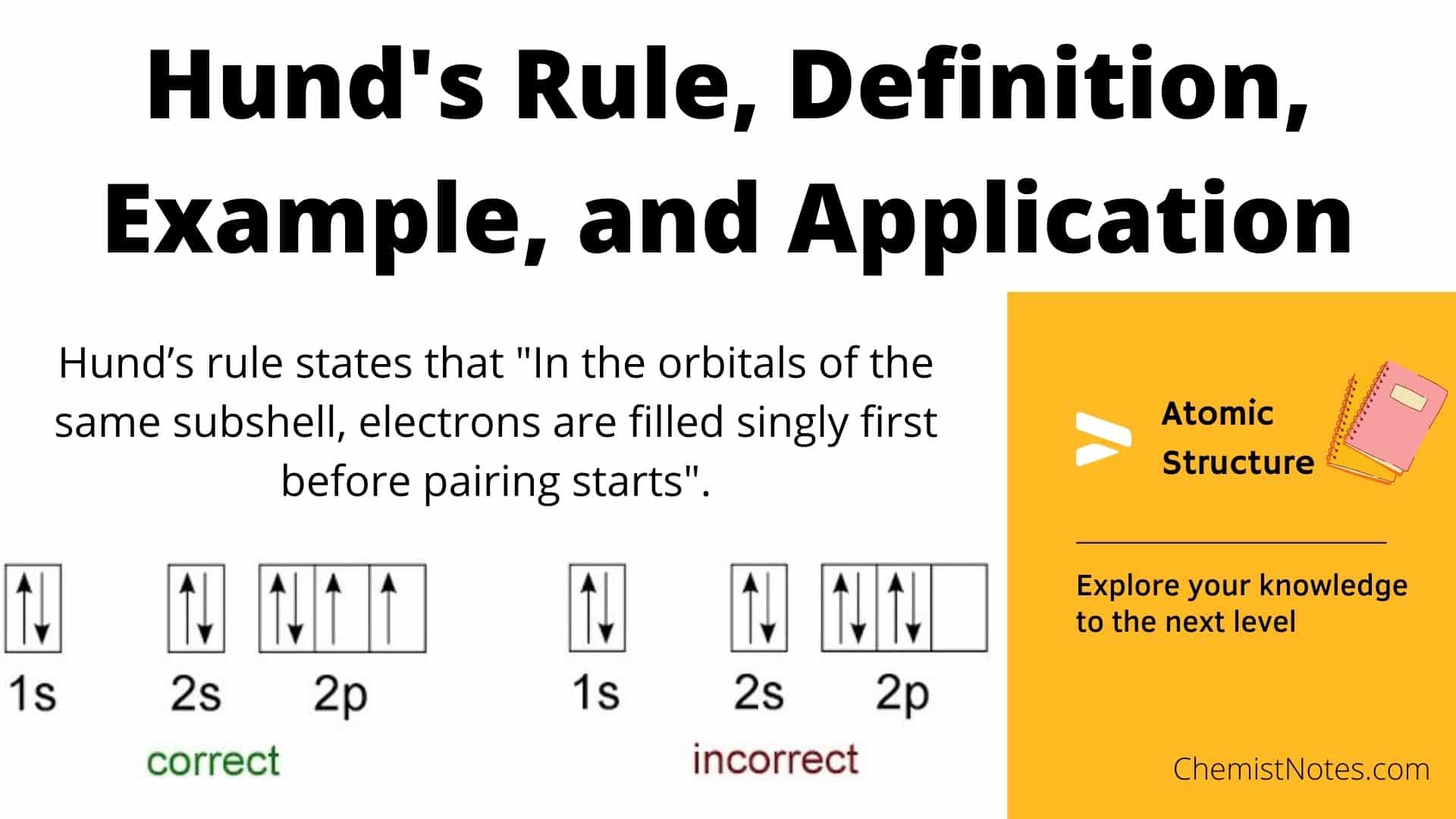 Source: chemistnotes.com
Source: chemistnotes.com
So the answer is b. Question 4 The electrons in the half-filled 4d orbitals dont all have the same spin. Which of the following diagrams best represents the scale of earth in comparison to a. Such arrangements of electrons in orbitals violate Hunds rule. Electron Configuration and its Purpose The electrical configuration of an atom is the distribution of electrons into orbitals.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The Pauli Exclusion Principle states that no two electrons can be identified by the same set of quantum numbers. Ignoring the Hund principle the Aufbau principle states that electrons have to first fill the lowest energy levels. According to Hunds rule the two 2s electrons will occupy the same orbital but the two 2p electrons will occupy distinct orbitals. Recognizing examples and non-examples of Hunds Rule - YouTube. You have two electrons in one 2p orbital but none in the other 2p orbitals.
 Source: study.com
Source: study.com
There must be one electron with the same spin in each orbital of the same energy before you can put two in the same orbital. The Pauli Exclusion Principle states that no two electrons can be identified by the same set of quantum numbers. So the answer is b. The image attached is the example of hunds rule. This violates Hunds Rule.
 Source: chemistrylearner.com
Source: chemistrylearner.com
For example for boron through neon the electron filling order of the 2p orbitals follows Hunds Rule. There must be one electron with the same spin in each orbital of the same energy before you can put two in the same orbital. Similarly in the second figure 2Pz orbital is empty while 2P x and 2P y orbital are filled with two electrons. Which electron configuration represents a violation of Hunds Rule. How many electrons can be described by the set of quantum numbers n 4 l 2ml 0 ms ½.
 Source: youtube.com
Source: youtube.com
This violates Hunds Rule. The Pauli Exclusion Principle states that no two electrons can be identified by the same set of quantum numbers. Why do copper and chromium violates the Aufbau principle. Use Hunds rule to derive the electronic configuration of Ce3 ion and calculate its magnetic moment on the basis of spin-only formula. Hunds rule of maximum multiplicity is a rule based on observation of atomic spectra which is used to predict the ground state of an atom or molecule with one or more open electronic shells.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title violation of hunds rule example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.