Your Vaccine lot number example images are available in this site. Vaccine lot number example are a topic that is being searched for and liked by netizens today. You can Download the Vaccine lot number example files here. Download all royalty-free vectors.
If you’re looking for vaccine lot number example pictures information related to the vaccine lot number example keyword, you have visit the right blog. Our website frequently provides you with hints for viewing the maximum quality video and picture content, please kindly hunt and find more enlightening video content and graphics that match your interests.
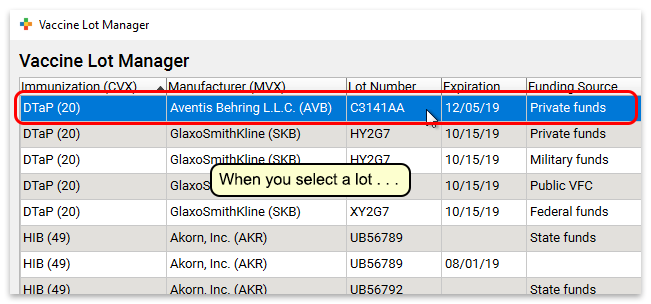
Vaccine Lot Number Example. Non-decremented doses can be discovered via the Patient Detail Report. LOT B-01580609 means that the product was manufactured on February 15 2008 and the earliest expiration date for any item in the kit is June 2009. Lot number - P332D is a non-decremented dose. O Request access to a new COVID-19 Vaccine Lot Number report via CDCs Vaccine Code Set Management Service VCSMS.
 Vendors Roll Out Tracking Tools To Help Hr Comply With Osha Vaccine Rule From shrm.org
Vendors Roll Out Tracking Tools To Help Hr Comply With Osha Vaccine Rule From shrm.org
Check your vaccine stock using the CDCs Vaccine Lot Number and Expiration Date webpage. The 4th character changes to represent either the carton vial or oral applicator. 025 mL for age 6 months through 35 months 05 mL for age 3 years or older Route. Centers for Disease Control and Prevention. Small pharmacies may. This report includes COVID-19 vaccine lot numbers and expiration dates provided to CDC by the vaccine manufacturers.
Centers for Disease Control and Prevention.
2 The lot number and expiration date The lot number is a string of numbers and letters that tracks this specific batch of vaccine. The lot number can usually be found on the vaccine label or accompanying packaging. Check your vaccine stock using the CDCs Vaccine Lot Number and Expiration Date webpage. This report includes COVID-19 vaccine lot numbers and expiration dates provided to CDC by the vaccine manufacturers. Centers for Disease Control and Prevention. Non-decremented doses can be discovered via the Patient Detail Report.
 Source: npr.org
Source: npr.org
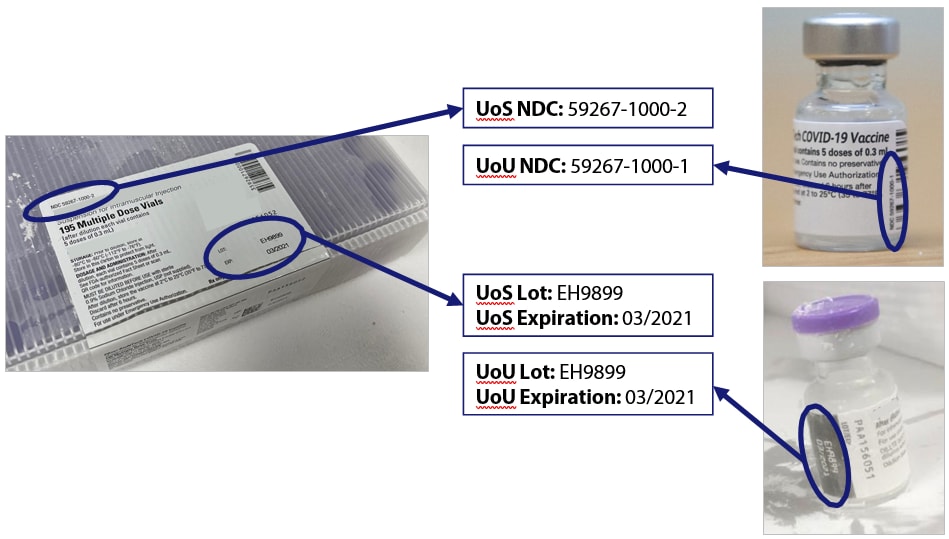
And for liquid diluent each with different NDCs and different lot numbers. LOT B-01580609 means that the product was manufactured on February 15 2008 and the earliest expiration date for any item in the kit is June 2009. The 4th character changes to represent either the carton vial or oral applicator. Vaccines manufactured by MedImmune AstraZeneca Pfizer Merck and Bio CSL have the same lot numbers on their UoS and UoU. 2021 2022 Influenza Season Vaccine Labels 3 Afluria Quadrivalent IIV4 Quadrivalent Inactivated Influenza Vaccine 5 mL Multi-dose Vial Ages.
 Source: cnbc.com
Source: cnbc.com
The 4th character changes to represent either the carton vial or oral applicator. However if numbers arent matching and you just cant find it you should not reconcile these out until youve contacted me. The lot number can usually be found on the vaccine label or accompanying packaging. Composition Lot Release and Other Seasonal Information Influenza Vaccine for the. The table produced by the American Immunization Registry Association AIRA shows the patterns for the different lot numbers for the six VFC vaccines this difference impacts.
 Source: cnbc.com
Source: cnbc.com
025 mL for age 6 months through 35 months 05 mL for age 3 years or older Route. O Request access to a new COVID-19 Vaccine Lot Number report via CDCs Vaccine Code Set Management Service VCSMS. If your inventory is updated using the lot number on the packing list the lot number shown is the. Pfizer and Moderna are two-dose vaccines while JJ is one dose. Please refer to the Patient Detail Report Guidelines for reference.
 Source: kcra.com
Source: kcra.com
A VIS was developed for MMRV. Wwwcdcgov vaccines. Composition Lot Release and Other Seasonal Information Influenza Vaccine for the. When more than 1 Symptom occurs in a single report then the percentage of Symptoms to unique events is more than 100. However if numbers arent matching and you just cant find it you should not reconcile these out until youve contacted me.

If you know you dropped and broke a vial or if a dose is legitimately expired you may reconcile it out using the appropriate category and reason. The table produced by the American Immunization Registry Association AIRA shows the patterns for the different lot numbers for the six VFC vaccines this difference impacts. The 4th character changes to represent either the carton vial or oral applicator. Non-decremented doses can be discovered via the Patient Detail Report. Home Page - COVID-19 VACCINE LOT NUMBER AND EXPIRATION DATES.
 Source: abc7chicago.com
Source: abc7chicago.com
These doses must be accounted for. The lot number can usually be found on the vaccine label or accompanying packaging. As the current Fact Sheet allows for 30-day storage at 5C please be advised that this specific lot should only be stored at 5C -3C for 7 days or April 28 whichever occurs first. Moderna COVID-19 Vaccine drug product Lot number 028A21A has been Quality released and assigned a Shelf Life Expiration Date of 28 APR 2021. All cards contain the lot number of the vaccine administered.
 Source: cbsnews.com
Source: cbsnews.com
Home Page - COVID-19 VACCINE LOT NUMBER AND EXPIRATION DATES. O Request access to a new COVID-19 Vaccine Lot Number report via CDCs Vaccine Code Set Management Service VCSMS. And for liquid diluent each with different NDCs and different lot numbers. When more than 1 Symptom occurs in a single report then the percentage of Symptoms to unique events is more than 100. The lot number can usually be found on the vaccine label or accompanying packaging.
 Source: washingtonpost.com
Source: washingtonpost.com
For example Pentacel has one NDC for the lyophilized freeze-dried powder component and a different NDC for the diluent. The numbers in each 10 pack are linked. Check your vaccine stock using the CDCs Vaccine Lot Number and Expiration Date webpage. For more information see Multiple Mentions. When more than 1 Symptom occurs in a single report then the percentage of Symptoms to unique events is more than 100.
 Source: nationalworld.com
Source: nationalworld.com
Last Name Date Of birth Product NameMan ufacturer Vaccine Lot Number Dose COVID-19 2nd Dose COVID-19 Other Other First Name Patient number medical record or IIS record number. If the multi-vaccine VIS is unavailable you should use the individual vaccine VISs. This report is updated daily and can be used to support vaccine administration inventory. Moderna will not be updating the Fact Sheet as this. Please refer to the Patient Detail Report Guidelines for reference.
 Source: ktla.com
Source: ktla.com
The applicant generally must submit samples of the product from the lot in question in order to permit the Agency to perform confirmatory testing. If you know you dropped and broke a vial or if a dose is legitimately expired you may reconcile it out using the appropriate category and reason. For example the number of Symptoms mentioned is likely to exceed the number of events reported because many reports include more than 1 Symptom. NA HL7 Data Type. Vaccines manufactured by MedImmune AstraZeneca Pfizer Merck and Bio CSL have the same lot numbers on their UoS and UoU.

The applicant generally must submit samples of the product from the lot in question in order to permit the Agency to perform confirmatory testing. 2021 2022 Influenza Season Vaccine Labels 3 Afluria Quadrivalent IIV4 Quadrivalent Inactivated Influenza Vaccine 5 mL Multi-dose Vial Ages. Pfizer and Moderna are two-dose vaccines while JJ is one dose. Search for the Patient using either the. As the current Fact Sheet allows for 30-day storage at 5C please be advised that this specific lot should only be stored at 5C -3C for 7 days or April 28 whichever occurs first.
 Source: cdc.gov
Source: cdc.gov
Rotarix Lot Number Configuration Example. Last Name Date Of birth Product NameMan ufacturer Vaccine Lot Number Dose COVID-19 2nd Dose COVID-19 Other Other First Name Patient number medical record or IIS record number. Check your vaccine stock using the CDCs Vaccine Lot Number and Expiration Date webpage. Have a different lot number on the UoS and UoU. Influenza Virus Vaccine Composition and Lot Release information older than 2 years is available on FDA Archive.
 Source: learn.pcc.com
Source: learn.pcc.com
All cards contain the lot number of the vaccine administered. If your inventory is updated using the lot number on the packing list the lot number shown is the. Have a different lot number on the UoS and UoU. 6 months or older Dosage. A lot number is a number given to a specific batch as it was manufactured and is used by the vaccine.

Saving Lives Protecting People. Small pharmacies may. Last Name Date Of birth Product NameMan ufacturer Vaccine Lot Number Dose COVID-19 2nd Dose COVID-19 Other Other First Name Patient number medical record or IIS record number. A lot number is a number given to a specific batch as it was manufactured and is used by the vaccine. The applicant generally must submit samples of the product from the lot in question in order to permit the Agency to perform confirmatory testing.
 Source: pna.gov.ph
Source: pna.gov.ph
The applicant generally must submit samples of the product from the lot in question in order to permit the Agency to perform confirmatory testing. A VIS was developed for MMRV. Centers for Disease Control and Prevention. Rotarix Lot Number Configuration Example. All cards contain the lot number of the vaccine administered.
 Source: abc7chicago.com
Source: abc7chicago.com
When more than 1 Symptom occurs in a single report then the percentage of Symptoms to unique events is more than 100. A lot number is a number given to a specific batch as it was manufactured and is used by the vaccine. Small pharmacies may. For example Pentacel has one NDC for the lyophilized freeze-dried powder component and a different NDC for the diluent. Vaccines manufactured by MedImmune AstraZeneca Pfizer Merck and Bio CSL have the same lot numbers on their UoS and UoU.
 Source: shrm.org
Source: shrm.org
When more than 1 Symptom occurs in a single report then the percentage of Symptoms to unique events is more than 100. 6 months or older Dosage. If your inventory is updated using the lot number on the packing list the lot number shown is the. Centers for Disease Control and Prevention. As the current Fact Sheet allows for 30-day storage at 5C please be advised that this specific lot should only be stored at 5C -3C for 7 days or April 28 whichever occurs first.

For certain combination vaccines given to children you can use the multi-vaccine VIS which includes DTaP Hib HepB polio and PCV13 and check the appropriate boxes just as you would if you were administering the individual vaccines. And for liquid diluent each with different NDCs and different lot numbers. All cards contain the lot number of the vaccine administered. LOT B-01580609 means that the product was manufactured on February 15 2008 and the earliest expiration date for any item in the kit is June 2009. Influenza Virus Vaccine Composition and Lot Release information older than 2 years is available on FDA Archive.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vaccine lot number example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






