Your Solid in solid solution example images are ready in this website. Solid in solid solution example are a topic that is being searched for and liked by netizens today. You can Download the Solid in solid solution example files here. Download all royalty-free photos and vectors.
If you’re searching for solid in solid solution example images information linked to the solid in solid solution example keyword, you have visit the ideal blog. Our site frequently provides you with suggestions for refferencing the highest quality video and picture content, please kindly search and locate more enlightening video articles and graphics that fit your interests.
Solid In Solid Solution Example. There are some examples of solution that are not liquid. A typical example of substitutional solid solution of Zn in Cu is observed in brass whereas an interstitial solid solution of C in. The example is derived from a diagram presented in Glynn and Parkhurst 1992. Eg solution of Gas solute in Gas solvent EXAMPLE Air.
 Advanced Distributed Learning Mobile Learning Instructional Design Learning Technology From pinterest.com
Advanced Distributed Learning Mobile Learning Instructional Design Learning Technology From pinterest.com
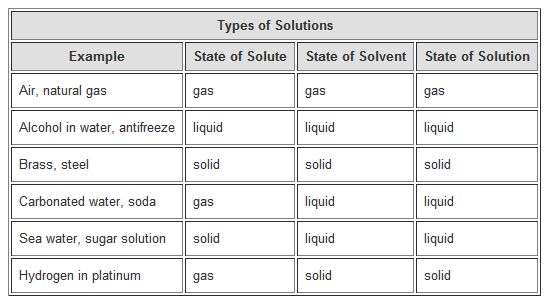
Solid solutions may be distinguished as substitutional when the volume of constituent elements is similar and interstitial when a volume difference size factor higher than 15 is observed. An example of solid solution is. One example of a liquid dissolved into a solid is dental amalgam. Take a look at the definition of substitutional solid solutions Hume-Rothery rules and. Stainless steel Stainless steel - Wikipedia is an alloy which is simply a special case of solid solution where a metal iron is the main component solvent and another element is. There are many examples of solidliquid solutions in everyday life.
Sports drinks - Sports drinks like Gatorade and Powerade are solutions of salt sugar and other ingredients dissolved in water.
Sugar water is an example of a solid-liquid solution. Homogeneous mixture can be formed in many ways. In some circumstances it is also possible to create a solution from a liquid solute and a solid solvent. It is not necessary that solution is always form between solid in liquid or liquid in liquid. Suspensions are called to those that do not reach the state of dissolution because the particles of the solid can be seen with the. Steel is comprised of iron and carbon.
 Source: pinterest.com
Source: pinterest.com
When a solid dissolves the solid solute and the liquid solvent form a very close intimate mixture called a solution. Take a look at the definition of substitutional solid solutions Hume-Rothery rules and. There are many examples of solidliquid solutions in everyday life. Solid solutions may be distinguished as substitutional when the volume of constituent elements is similar and interstitial when a volume difference size factor higher than 15 is observed. Pancake syrup - The syrup that you like to eat on pancakes or waffles is a solution of sugar in water along with flavoring agents.
 Source: pinterest.com
Source: pinterest.com
Solid-state solution of one or more solutes in a solvent. It is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes. Homogeneous mixture can be formed in many ways. Answer 1 of 2. To solve this question we need to classify each of the options based on the dispersed phase and the dispersion medium present in them.
 Source: pinterest.com
Source: pinterest.com
Dissolving mercury which is liquid at room temperature into silver forms this type of amalgam. An example of solid solution is. The following example considers an aragonite CaCO 3-strontianite SrCO 3 solid solution and demonstrates how the composition of the solid solution and aqueous phase change as strontium carbonate is added to an initially pure calcium carbonate system. One example of a liquid dissolved into a solid is dental amalgam. Some solid solutions examples are alloys that are a combination of two or more metals.
 Source: pinterest.com
Source: pinterest.com
Solid solutions may be distinguished as substitutional when the volume of constituent elements is similar and interstitial when a volume difference size factor higher than 15 is observed. Solutions They will be solutions if the formation is produced by the breakdown of the solid to the molecular or ionic level. In some circumstances it is also possible to create a solution from a liquid solute and a solid solvent. To form a solution the solute and solvent must have similar polarity. It is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes.
 Source: pinterest.com
Source: pinterest.com
There are many examples of solidliquid solutions in everyday life. Solid sol is the colloidal dispersion which is solid in the state but is little soft. Steel is comprised of iron and carbon. Sports drinks - Sports drinks like Gatorade and Powerade are solutions of salt sugar and other ingredients dissolved in water. Stainless steel Stainless steel - Wikipedia is an alloy which is simply a special case of solid solution where a metal iron is the main component solvent and another element is.
 Source: in.pinterest.com
Source: in.pinterest.com
Any object sink cooking ustensil made of stainless steel. Answer 1 of 2. Solid solution mixture of two crystalline solids that coexist as a new crystalline solid or crystal latticeThe mixing can be accomplished by combining the two solids when they have been melted into liquids at high temperatures and then cooling the result to form the new solid or by depositing vapours of the starting materials onto substrates to form thin films. To form a solution the solute and solvent must have similar polarity. Suspensions are called to those that do not reach the state of dissolution because the particles of the solid can be seen with the.
 Source: br.pinterest.com
Source: br.pinterest.com
An example of solid solution is. Solid solution mixture of two crystalline solids that coexist as a new crystalline solid or crystal latticeThe mixing can be accomplished by combining the two solids when they have been melted into liquids at high temperatures and then cooling the result to form the new solid or by depositing vapours of the starting materials onto substrates to form thin films. Homogeneous mixtures can be solid liquid or gas but in order to be homogeneous or form a solution they must be of uniform state once mixed. Solution is form when solute is dissolve in solvent. There are many examples of solidliquid solutions in everyday life.
 Source: pinterest.com
Source: pinterest.com
Just as sugar dissolved in water is a liquid solution carbon dissolved in iron is an interstitial solid solution. Solution is form when solute is dissolve in solvent. Homogeneous mixtures can be solid liquid or gas but in order to be homogeneous or form a solution they must be of uniform state once mixed. Steel is comprised of iron and carbon. Any object sink cooking ustensil made of stainless steel.
 Source: pinterest.com
Source: pinterest.com
Solution - A solution is a mixture formed when a solid liquid or gaseous substance is homogeneously mixed with a liquid. Gemstones are the example of solid sol. A typical example of substitutional solid solution of Zn in Cu is observed in brass whereas an interstitial solid solution of C in. Sports drinks - Sports drinks like Gatorade and Powerade are solutions of salt sugar and other ingredients dissolved in water. It is not necessary that solution is always form between solid in liquid or liquid in liquid.
 Source: in.pinterest.com
Source: in.pinterest.com
Solutions They will be solutions if the formation is produced by the breakdown of the solid to the molecular or ionic level. Brass is combination of. An example of solid solution is. The example is derived from a diagram presented in Glynn and Parkhurst 1992. A few other interesting examples of interstitial solid solutions are.
 Source: pinterest.com
Source: pinterest.com
Steel is comprised of iron and carbon. Answer 1 of 2. Some solid solutions examples are alloys that are a combination of two or more metals. Solution is form when solute is dissolve in solvent. A typical example of substitutional solid solution of Zn in Cu is observed in brass whereas an interstitial solid solution of C in.
 Source: pinterest.com
Source: pinterest.com
Stainless steel Stainless steel - Wikipedia is an alloy which is simply a special case of solid solution where a metal iron is the main component solvent and another element is. Just as sugar dissolved in water is a liquid solution carbon dissolved in iron is an interstitial solid solution. Gemstones are the example of solid sol. It is frequent that the solids that are part of the solutions react well in some solutes and badly in others. Solution of solid solute in solid solvent EXAMPLE carbon in iron or steel.
 Source: pinterest.com
Source: pinterest.com
Suspensions are called to those that do not reach the state of dissolution because the particles of the solid can be seen with the. Solution of solid solute in solid solvent EXAMPLE carbon in iron or steel. Solid Sol is a colloid in which solid particles are dispersed in a solid medium. Solutions They will be solutions if the formation is produced by the breakdown of the solid to the molecular or ionic level. Solids are colloidal solutions in which both the dispersed phase and the dispersion medium are solids.
 Source: pinterest.com
Source: pinterest.com
Eg solution of Gas solute in Gas solvent EXAMPLE Air. An example of solid solution is. Gemstones are the example of solid sol. A substitutional solid solution is a type of solution where two different atom types can switch. Solid solutions may be distinguished as substitutional when the volume of constituent elements is similar and interstitial when a volume difference size factor higher than 15 is observed.
 Source: pinterest.com
Source: pinterest.com
There are some examples of solution that are not liquid. Solution - A solution is a mixture formed when a solid liquid or gaseous substance is homogeneously mixed with a liquid. Solid solution mixture of two crystalline solids that coexist as a new crystalline solid or crystal latticeThe mixing can be accomplished by combining the two solids when they have been melted into liquids at high temperatures and then cooling the result to form the new solid or by depositing vapours of the starting materials onto substrates to form thin films. The example is derived from a diagram presented in Glynn and Parkhurst 1992. Likewise a solvent is a substance in which another substance dissolves.
 Source: pinterest.com
Source: pinterest.com
Solution is form when solute is dissolve in solvent. The most common examples of dissolving involve a solid and a liquid usually water. Sports drinks - Sports drinks like Gatorade and Powerade are solutions of salt sugar and other ingredients dissolved in water. Take a look at the definition of substitutional solid solutions Hume-Rothery rules and. Solution is form when solute is dissolve in solvent.
 Source: pinterest.com
Source: pinterest.com
A typical example of substitutional solid solution of Zn in Cu is observed in brass whereas an interstitial solid solution of C in. Suspensions are called to those that do not reach the state of dissolution because the particles of the solid can be seen with the. To learn more about Properties Types Videos. Brass is combination of. Solutions They will be solutions if the formation is produced by the breakdown of the solid to the molecular or ionic level.
 Source: pinterest.com
Source: pinterest.com
Solutions They will be solutions if the formation is produced by the breakdown of the solid to the molecular or ionic level. Any object sink cooking ustensil made of stainless steel. Sugar water is an example of a solid-liquid solution. There are some examples of solution that are not liquid. The following example considers an aragonite CaCO 3-strontianite SrCO 3 solid solution and demonstrates how the composition of the solid solution and aqueous phase change as strontium carbonate is added to an initially pure calcium carbonate system.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title solid in solid solution example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.