Your Reduction half reaction example images are available in this site. Reduction half reaction example are a topic that is being searched for and liked by netizens now. You can Download the Reduction half reaction example files here. Get all free photos and vectors.
If you’re looking for reduction half reaction example pictures information linked to the reduction half reaction example topic, you have pay a visit to the right blog. Our site always gives you suggestions for seeing the highest quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
Reduction Half Reaction Example. One of the basic reasons that the concept of oxidation-reduction reactions helps to correlate chemical knowledge is that a particular oxidation or reduction can often be carried out by a wide variety of oxidizing or reducing agents. Redox reactions can be considered to have two hypothetical half-reactions. But thanks to the redox reaction calculator which makes it easier for students and researchers to balance a complicated redox equation in just a second. Leo the lion goes gerLeo.
 How To Balance Redox Equations In Basic Solution College Chemistry Chemistry Equations From pinterest.com
How To Balance Redox Equations In Basic Solution College Chemistry Chemistry Equations From pinterest.com
Label each as oxidation or reduction. There will be times when you want to switch a half-reaction from one of the two types to the other. Oidation half-reaction 74 MnO MnO4. In this case the lowest common multiple is 6 so we need to multiply the reduction half equation by 2 and the oxidation half equation by 3. The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction. Use the Activity Series Chart.
Since the process of oxidation-reduction involves the addition and removal of atoms and charges writing a balanced chemical equation for such reactions is quite a daunting task.
In the following redox reaction which species is being oxidized. Now both half-reactions are balanced and the total charges of each side are equal. Als is being oxidized. The reduction potential is a direct measure of the thermodynamic feasibility of an oxidationreduction half-reaction. And it is fundamentally important in many aspects of organic bioinorganic and environmental chemistry as well as in biology and materials science. 2 reduction half-reaction.
 Source: es.pinterest.com
Source: es.pinterest.com
Als is being oxidized. The following examples show a typical redox reaction with its elements 1. A mnemonic you might find helpful to remember the definitions of oxidation and reduction is. In the following redox reaction which species is being oxidized. Lets have this reaction as an example.
 Source: pinterest.com
Source: pinterest.com
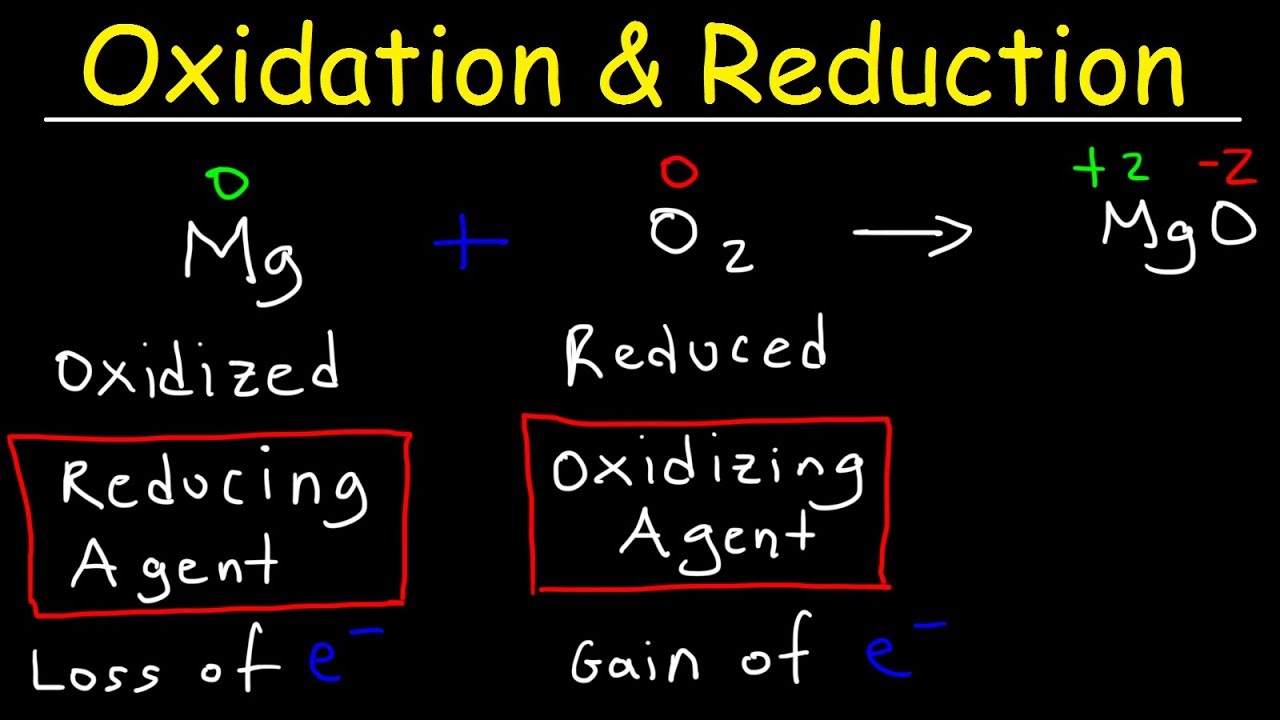
The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction. Oxidation is loss of ereduction is gain of e a Oxidation b Reduction c Oxidizing agent d Reducing agent 2. When natural gas burns for example an oxidation-reduction reaction occurs that releases more than 800 kJmol of energy. Reaction between Iron and Hydrogen Peroxide. Now both half-reactions are balanced and the total charges of each side are equal.
 Source: se.pinterest.com
Source: se.pinterest.com
Reduction-oxidation reactions are often called redox equations. Cu 2 2e Cu. We then balance the half-reactions one at a time and combine them so that electrons are neither created nor destroyed in the reaction. A powerful technique for balancing oxidation-reduction equations involves dividing these reactions into separate oxidation and reduction half-reactions. The oxidation and reduction reaction also involve the addition of oxygen or hydrogen to different substances.
 Source: in.pinterest.com
Source: in.pinterest.com
When K 2 Cr 2 O 7 aq orange is acidified with H 2 SO 4 aq and reacted with H 2 O 2 aq colorless the solution turns green indicating formation of Cr3 and bubbles are formed indicating the formation of O 2 g. The sum of both half reactions maintains the charge neutrality of the original reaction. The Half-Reaction Method of Balancing Redox Equations. Label each as oxidation or reduction. In this case the lowest common multiple is 6 so we need to multiply the reduction half equation by 2 and the oxidation half equation by 3.
 Source: pinterest.com
Source: pinterest.com
Lets have this reaction as an example. Examples of Reduction. 2 reduction half-reaction. Reduction-oxidation reactions are often called redox equations. When K 2 Cr 2 O 7 aq orange is acidified with H 2 SO 4 aq and reacted with H 2 O 2 aq colorless the solution turns green indicating formation of Cr3 and bubbles are formed indicating the formation of O 2 g.
 Source: pinterest.com
Source: pinterest.com
A powerful technique for balancing oxidation-reduction equations involves dividing these reactions into separate oxidation and reduction half-reactions. Which one is being reduced. 1 0 I I2. 2 reduction half-reaction. Use the Activity Series Chart.
 Source: pinterest.com
Source: pinterest.com
Zn Zn 2 2e The reduction half-reaction can be written as. The oxidation and reduction reaction also involve the addition of oxygen or hydrogen to different substances. The sum of both half reactions maintains the charge neutrality of the original reaction. Zn s 2H aq Zn 2 aq H 2 g Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium oxide. The following examples show a typical redox reaction with its elements 1.
 Source: pinterest.com
Source: pinterest.com
Reaction between Iron and Hydrogen Peroxide. Reaction between Iron and Hydrogen Peroxide. 1 0 I I2. We find examples of oxidation-reduction or redox reactions almost every time we analyze the reactions used as sources of either heat or work. Therefore three electrons must be added to the left side to equalize the charges.
 Source: pinterest.com
Source: pinterest.com
There is one I atom on the left-side and two I atoms on the right-side. Leo the lion goes gerLeo. Oxidation and Reduction reactions- The chemical reactions which involve the transfer of electrons from one chemical substance to another. Break the above given equation into two half-equations. The sum of both half reactions maintains the charge neutrality of the original reaction.
 Source: pinterest.com
Source: pinterest.com
In the reduction half-reaction the total charge of the right side is zero and the left side is 3. Since the process of oxidation-reduction involves the addition and removal of atoms and charges writing a balanced chemical equation for such reactions is quite a daunting task. One of the basic reasons that the concept of oxidation-reduction reactions helps to correlate chemical knowledge is that a particular oxidation or reduction can often be carried out by a wide variety of oxidizing or reducing agents. But thanks to the redox reaction calculator which makes it easier for students and researchers to balance a complicated redox equation in just a second. The reduction potential is a direct measure of the thermodynamic feasibility of an oxidationreduction half-reaction.
 Source: pinterest.com
Source: pinterest.com
Oidation half-reaction 74 MnO MnO4. There will be times when you want to switch a half-reaction from one of the two types to the other. Lets have this reaction as an example. Note that when we look at reactions this way we do not need to remember which half reaction one. In the following redox reaction which species is being oxidized.
 Source: pinterest.com
Source: pinterest.com
Als is being oxidized. Examples of Reduction. Oxidation and reduction half reactions. Lets have this reaction as an example. Oidation half-reaction 74 MnO MnO4.
 Source: pinterest.com
Source: pinterest.com
The reduction potential is a direct measure of the thermodynamic feasibility of an oxidationreduction half-reaction. Therefore three electrons must be added to the left side to equalize the charges. Now both half-reactions are balanced and the total charges of each side are equal. K 2 Cr 2 O 7. These electron-transfer reactions are termed as oxidation-reduction reactions or Redox reactions.
 Source: pinterest.com
Source: pinterest.com
Leo the lion goes gerLeo. Redox calculator is an online tool which you can. The reduction potential is a direct measure of the thermodynamic feasibility of an oxidationreduction half-reaction. Hence I atoms have to be balanced by. The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction.
 Source: pinterest.com
Source: pinterest.com
Then reaction 1 and the reversed reaction 2 can be added together to arrive at the net redox reaction and the D E can be found by adding the reduction potential of the first half-reaction to the oxidation potential of the second half reaction. A mnemonic you might find helpful to remember the definitions of oxidation and reduction is. Using the half reaction table above split the following biochemical redox reaction into its constituent half reactions. Hence I atoms have to be balanced by. Then reaction 1 and the reversed reaction 2 can be added together to arrive at the net redox reaction and the D E can be found by adding the reduction potential of the first half-reaction to the oxidation potential of the second half reaction.
 Source: pinterest.com
Source: pinterest.com
Fe 2 is oxidized to Fe 3 by hydrogen peroxide when an acid is. When natural gas burns for example an oxidation-reduction reaction occurs that releases more than 800 kJmol of energy. Cu 2 2e Cu. Oxidation is loss of ereduction is gain of e a Oxidation b Reduction c Oxidizing agent d Reducing agent 2. In this case the lowest common multiple is 6 so we need to multiply the reduction half equation by 2 and the oxidation half equation by 3.
 Source: pinterest.com
Source: pinterest.com
In this case the lowest common multiple is 6 so we need to multiply the reduction half equation by 2 and the oxidation half equation by 3. Reduction reactions always occur in conjunction with oxidation reactions in which a reactant loses one or more electrons. Lets have this reaction as an example. Reduction-oxidation reactions are often called redox equations. 1 0 I I2.
 Source: pinterest.com
Source: pinterest.com
Reduction-oxidation reactions are often called redox equations. Zn Zn 2 2e The reduction half-reaction can be written as. The redox reaction before step 0 is. Zn s 2H aq Zn 2 aq H 2 g Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium oxide. A Al b Ba2 c Br 2.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title reduction half reaction example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






