Your Polar covalent bond examples list images are ready in this website. Polar covalent bond examples list are a topic that is being searched for and liked by netizens today. You can Get the Polar covalent bond examples list files here. Get all royalty-free photos.
If you’re searching for polar covalent bond examples list images information connected with to the polar covalent bond examples list keyword, you have come to the right blog. Our website frequently gives you hints for downloading the maximum quality video and image content, please kindly search and find more informative video articles and graphics that match your interests.
Polar Covalent Bond Examples List. In this bond the chlorine atom spends more time with the electrons than the hydrogen atom. Covalent bonds can be non-polar or polar and react to electrostatic charges. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. If the difference is less than 05 the atoms normally form a non-polar covalent bond while ionic bonds are formed when the difference is greater than 20.
 Flashcards Polar Nonpolar Covalent Bonds List Flashcards Study Com From study.com
Flashcards Polar Nonpolar Covalent Bonds List Flashcards Study Com From study.com
Examples of Molecules with Polar Covalent Bonds. Below is a list of such molecules with their formulae. A bond between 2 nonmetal atoms that have the same electronegativity and therefore have equal sharing of the bonding electron pair. Examples of Covalent Bond. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220. In this atomic molecule two hydrogen atoms share their single electrons with the oxygen atom which shares its own two electrons in return.
The result is a polar covalent bond a bond with uneven distribution of electron density.
Luckily you can look up electronegativity on a table to foretell whether or not atoms are likely to form polar covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. This type of bond can only occur when the difference in electronegativity is between 04 and 20. A polar covalent bond is a very strong bond where electrons are shared between normally two nonmetal atoms which have a similar electronegativity. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. Polar covalent bond Hydrogen chloride also called hydrochloric acid is.
 Source: study.com
Source: study.com
Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220. Here is a table listing molecules with polar and non. There is a nonpolar covalent bond examples list below. The result is a polar covalent bond a bond with uneven distribution of electron density.
 Source: chemistrylearner.com
Source: chemistrylearner.com
If the electronegativity variation between the two atoms is between 05 and 20 the atoms form a polar covalent bond. Water H 2 O is a polar bonded molecule. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220. This occurs because some atoms like electrons more than others they are more electronegative. Another example of a polar covalent bond is between a hydrogen and a chlorine atom.
 Source: slideplayer.com
Source: slideplayer.com
The inequality in electron distribution accounts for the bent shape of the molecule. The oxygen side of the molecule has a net negative charge while the two. This happens when there is a difference between the electronegativity values of each atom. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. The inequality in electron distribution accounts for the best shape of the molecule.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Some nonpolar molecules also contain polar covalent bonds. Examples of Covalent Bond. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. The electronegativity value for oxygen is 344 whereas the electronegativity value for hydrogen is 220. Polar Molecules.
 Source: chemistrytalk.org
Source: chemistrytalk.org
The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220. Because of this. Water H2O is a molecule having a polar covalent bond. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. The oxygen side of the molecule features.
 Source: researchgate.net
Source: researchgate.net
Benzene C 6 H 6 Hydrogen H 2 Nitrogen N 2 Oxygen O 2 Chlorine Cl 2 Carbon Dioxide CO 2 All of these share equal electrons and show zero dipole moment. Polar Molecules. In this atomic molecule two hydrogen atoms share their single electrons with the oxygen atom which shares its own two electrons in return. In polar covalent bonds the electrons are. Helium He Bromine Br 2.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The degree of polarity of a bond particularly depends on the difference in electronegativities of the two atoms bonded together and partly on other factors such as the size of the atoms. Types of Covalent Bonds. Polar Molecules. Water H2O is a polar bonded molecule. Because of this.
 Source: shutterstock.com
Source: shutterstock.com
Water H2O is a molecule having a polar covalent bond. This happens when there is a difference between the electronegativity values of each atom. An example is water. The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible. Some nonpolar molecules also contain polar covalent bonds.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The bonds between hydrogen and oxygen in water are an example of this type of bond. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. Here is a table listing molecules with polar and non. Luckily you can look up electronegativity on a table to foretell whether or not atoms are likely to form polar covalent bonds. The difference in the distribution of electrons accounts for the best shape of the molecule.
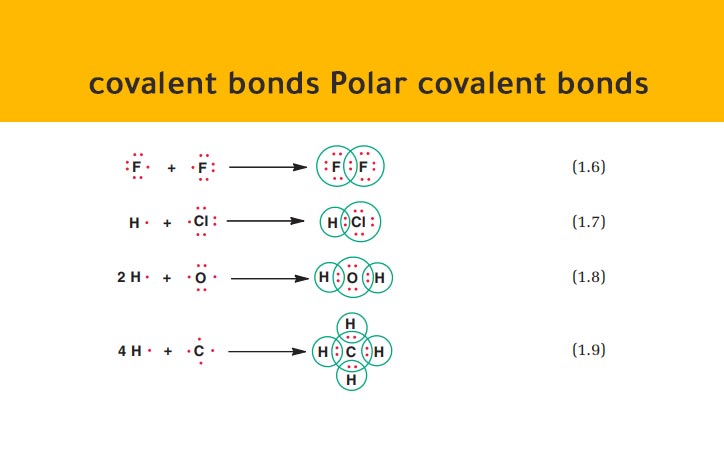 Source: chemistry1science.com
Source: chemistry1science.com
Polar covalent bond Hydrogen chloride also called hydrochloric acid is. A polar covalent bond is a very strong bond where electrons are shared between normally two nonmetal atoms which have a similar electronegativity. This occurs because some atoms like electrons more than others they are more electronegative. Examples of polar covalent bonds. Water H 2 O is a polar bonded molecule.

The difference in the distribution of electrons accounts for the best shape of the molecule. If the electronegativity variation between the two atoms is between 05 and 20 the atoms form a polar covalent bond. Water consists of a covalent bond containing hydrogen and oxygen bonding together to make H 2 O. Polar Covalent Bond Examples of Molecules with Polar Covalent Bond. The water H 2 O is the most classic example of a polar molecule.
 Source: chemistrylearner.com
Source: chemistrylearner.com
An example is water. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. Helium He Bromine Br 2. The difference in the distribution of electrons accounts for the best shape of the molecule. The inequality in electron distribution accounts for the bent shape of the molecule.
 Source: biologyfisiology.blogspot.com
Source: biologyfisiology.blogspot.com
The oxygen side of the molecule has a net negative charge while the two. The oxygen side of the molecule has a net negative charge while the two. Water H 2 O is a polar bonded molecule. Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms. Examples of Molecules with Polar Covalent Bonds.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible. Examples of Covalent Bond. The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220. This happens when there is a difference between the electronegativity values of each atom. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions.
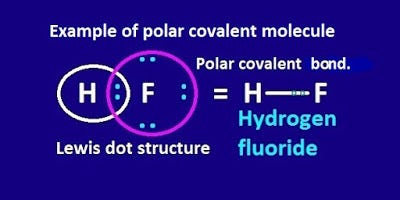 Source: kgghosh1990.medium.com
Source: kgghosh1990.medium.com
An absolute difference forms an ionic bond while a minor difference forms a polar covalent bond. Below is a list of such molecules with their formulae. The result is a polar covalent bond a bond with uneven distribution of electron density. Helium He Bromine Br 2. Such types of covalent bonds are Polar Covalent bonds.
 Source: slideplayer.com
Source: slideplayer.com
The result is a polar covalent bond a bond with uneven distribution of electron density. Polar Molecules. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. The result is a polar covalent bond a bond with uneven distribution of electron density.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The result is a polar covalent bond a bond with uneven distribution of electron density. A polar covalent bond occurs when atoms are shared unequally in a covalent bond. The result is a polar covalent bond a bond with uneven distribution of electron density. In this bond the chlorine atom spends more time with the electrons than the hydrogen atom. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge.

Hydrogen Molecule H2 is a non-polar covalent bond example as an electron pair is equally shared between the two hydrogen atoms. An example is water. A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. Nonpolar Covalent Bond Examples. The electronegativity value of oxygen is 344 while the electronegativity of hydrogen is 220.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title polar covalent bond examples list by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






