Your Pair of isotopes example images are ready in this website. Pair of isotopes example are a topic that is being searched for and liked by netizens now. You can Download the Pair of isotopes example files here. Get all free vectors.
If you’re looking for pair of isotopes example pictures information connected with to the pair of isotopes example topic, you have come to the ideal blog. Our site always gives you suggestions for refferencing the maximum quality video and picture content, please kindly surf and locate more informative video content and images that fit your interests.
Pair Of Isotopes Example. You will see here that the number of protons is the same in both the isotopes but. Which of the following pairs represent an isotope. What is an isotope. For example elements calcium and argon are isobars.
 Pin By Sabiha Khan On School In 2021 Linear Equations Equations Solutions From in.pinterest.com
Pin By Sabiha Khan On School In 2021 Linear Equations Equations Solutions From in.pinterest.com
CaZ 20. For example an isotope with 6 protons and 6 neutrons is carbon-12 or C-12. Isotopes are variants of a particular element with different a different number of neutrons. The mole and Avogadros number. Uranium-234 forms as a decay product. Counting protons electrons and neutrons.
For example the two isotopes of Uranium are 235 92 U and 239 92 U.
Radioactive isotopes may also be classified as stable isotopes when their half-lives are too long to be measured. Leading examples of isotopes. Uranium-234 forms as a decay product. The normal ones are carbon-12. Atomic number 17 Atomic number 17. An isotope with 6 protons and 7 neutrons is carbon-13 or C-16.
 Source: clutchprep.com
Source: clutchprep.com
Isotopes of an element are atoms of the same element with same number of protons but different number of neutrons. Learn this topic by watching Isotopes Concept Videos. Uranium-234 forms as a decay product. Is a carbon isotope with a half-life of 5730 years that is used in archeology to determine the age of rocks and organic matter. Examples of radiogenic isotopes include argon-40 and hydrogen-4.
 Source: pinterest.com
Source: pinterest.com
B Similarity A pair of isotopes have the same atomic number. An isotope with 6 protons and 7 neutrons is carbon-13 or C-16. Is a carbon isotope with a half-life of 5730 years that is used in archeology to determine the age of rocks and organic matter. 1 but different mass numbers. Write the electronic configuration of any one pair of isotopes and isobars.
 Source: in.pinterest.com
Source: in.pinterest.com
Lesson Summary The isotopes of an element are like different versions of an element - they have the same number of protons but. For example if an isotope has a -3 charge as with. What are isotope give example of pair of isotopes. Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. The normal ones are carbon-12.
 Source: pinterest.com
Source: pinterest.com
Isobars have same mass number but different atomic numbers. This uranium isotope is used in nuclear power plants to provide nuclear power just as it is used to build atomic bombs. Is a carbon isotope with a half-life of 5730 years that is used in archeology to determine the age of rocks and organic matter. B Give one similarity and one difference between a pair of isotopes. C 189 X 178 X.
 Source: youtube.com
Source: youtube.com
From the above notation to be the pair of isotope Z should be similar. For example the two isotopes of Uranium are 235 92 U and 239 92 U. Only choice b has 2 atoms of X with 6 protons and 8 and 6 neutrons respectively. Isotope Examples Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. D 1910 X 199 X.
 Source: pinterest.com
Source: pinterest.com
These elements can often be found to occur in nature and include isotopes of carbon nitrogen hydrogen oxygen noble gases and metals. CaZ 20. Other examples includes boron-12 and carbon-13 nuclei both contain 7 neutrons and so are isotones. The normal ones are carbon-12. Example 14 6 C 6 14 C 15 7 N 7 15 N.
 Source: in.pinterest.com
Source: in.pinterest.com
What is an isotope. Species having same number of neutrons but different number of protons are called Isotones. Click hereto get an answer to your question From the given examples form the pair of isotopes and the pair of isobars. Examples of radiogenic isotopes include argon-40 and hydrogen-4. Which pair of atoms constitutes a pair of isotopes of the same element.
 Source: pinterest.com
Source: pinterest.com
What is an isotope. Examples of radiogenic isotopes include argon-40 and hydrogen-4. Only choice b has 2 atoms of X with 6 protons and 8 and 6 neutrons respectively. Example 14 6 C 6 14 C 15 7 N 7 15 N. B 136 X 126 X.

A 158 X 157 X. Isotope Examples Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. Which pair of atoms constitutes a pair of isotopes of the same element. Only A and B have pairs of isotopes and one the other hand C and D have different atomic number. Counting protons electrons and neutrons.
 Source: pinterest.com
Source: pinterest.com
Atomic number mass number and isotopes. Uranium-234 forms as a decay product. C 189 X 178 X. Isotopes are variants of a particular element with different a different number of neutrons. The difference in mass number is due to the presence of a different number of neutrons in the elements.
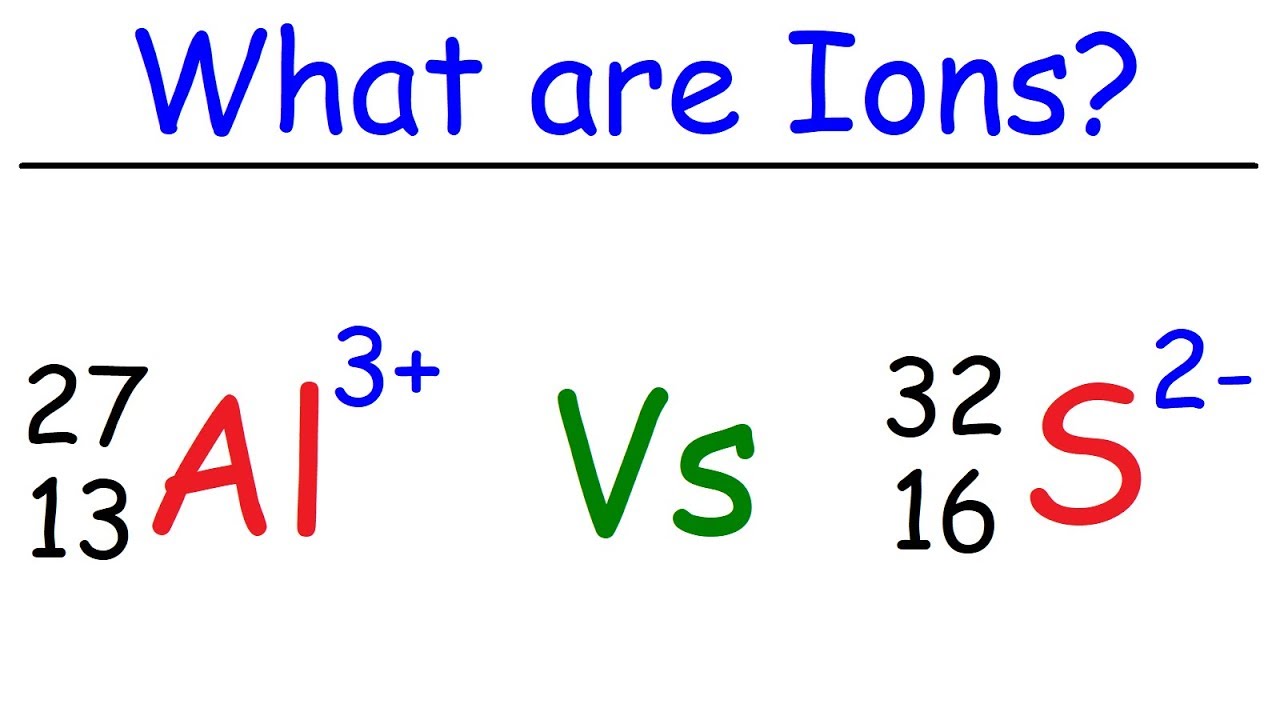 Source: youtube.com
Source: youtube.com
These elements can often be found to occur in nature and include isotopes of carbon nitrogen hydrogen oxygen noble gases and metals. Is a carbon isotope with a half-life of 5730 years that is used in archeology to determine the age of rocks and organic matter. Write the electronic configuration of any pair of isotopes and isobars. What is an example of a pair of isotopes. Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons.

Carbon-12 is a stable isotope while carbon-14 is a radioactive isotope radioisotope. 18Ar40 17Cl35 20Ca40 17Cl37. Click hereto get an answer to your question From the given examples form the pair of isotopes and the pair of isobars. Difference A pair of isotopes have different mass numbers. Carbon-12 is a stable isotope while carbon-14 is a radioactive isotope radioisotope.
 Source: youtube.com
Source: youtube.com
Leading examples of isotopes. Identifying isotopes and ions. Carbon-12 is a stable isotope while carbon-14 is a radioactive isotope radioisotope. Isotopes are variants of a particular element with different a different number of neutrons. B Similarity A pair of isotopes have the same atomic number.
 Source: pinterest.com
Source: pinterest.com
What is an example of a pair of isotopes. C Give the number of protons neutrons and electrons per atom in the two isotopes of chlorine 35 17 Cl and 37 17 Cl. 2 8 8 2 Ar Z 18. B 136 X 126 X. Ii Electronic configuration of pair of isobars.
 Source: pinterest.com
Source: pinterest.com
The normal ones are carbon-12. Ii Electronic configuration of pair of isobars. Which pair of atoms constitutes a pair of isotopes of the same element. C Give the number of protons neutrons and electrons per atom in the two isotopes of chlorine 35 17 Cl and 37 17 Cl. Similarly 36S 36 S 37Cl 37 C l 38Ar 38 A r 39K 39 K and 40Ca 40 C a nuclei are all isotones of 20.
 Source: pinterest.com
Source: pinterest.com
Here all three of them have same atomic number ie. Only choice b has 2 atoms of X with 6 protons and 8 and 6 neutrons respectively. For example there are a lot of carbon atoms in the universe. B Similarity A pair of isotopes have the same atomic number. Lesson Summary The isotopes of an element are like different versions of an element - they have the same number of protons but.
 Source: pinterest.com
Source: pinterest.com
The usefulness of a particular isotope pair is dependent upon the natural abundance of the parent element and whether the sample contains the parent. The mole and Avogadros number. Isobars have same mass number but different atomic numbers. Isotopes are defined as those chemical species which have the same atomic number but a different mass number. Isotopes of an element are atoms of the same element with same number of protons but different number of neutrons.
 Source: clutchprep.com
Source: clutchprep.com
Click hereto get an answer to your question From the given examples form the pair of isotopes and the pair of isobars. Identifying isotopes and ions. Mass number 35 Mass number 37. Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. Write the electronic configuration of any one pair of isotopes and isobars.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title pair of isotopes example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.