Your Examples of conformational isomers images are available in this site. Examples of conformational isomers are a topic that is being searched for and liked by netizens today. You can Get the Examples of conformational isomers files here. Find and Download all royalty-free photos and vectors.
If you’re looking for examples of conformational isomers images information linked to the examples of conformational isomers interest, you have pay a visit to the right blog. Our site always gives you hints for viewing the maximum quality video and picture content, please kindly hunt and find more enlightening video articles and images that match your interests.
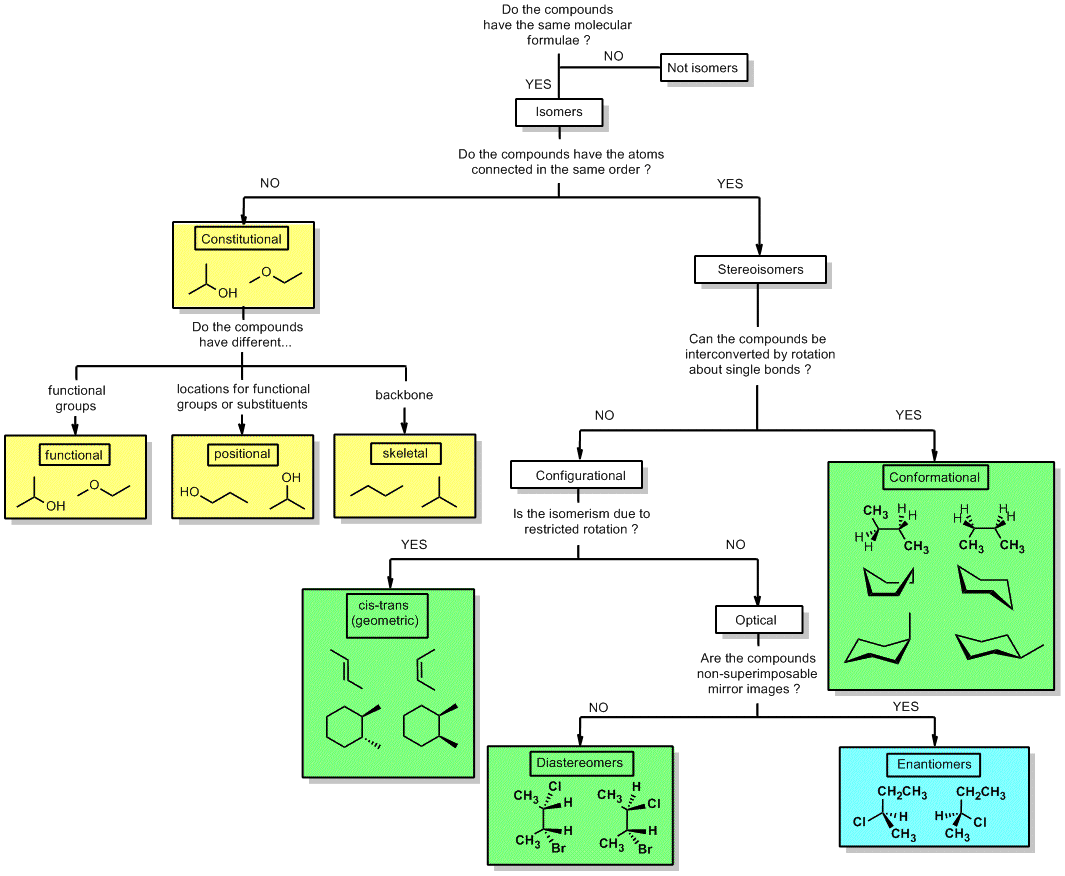
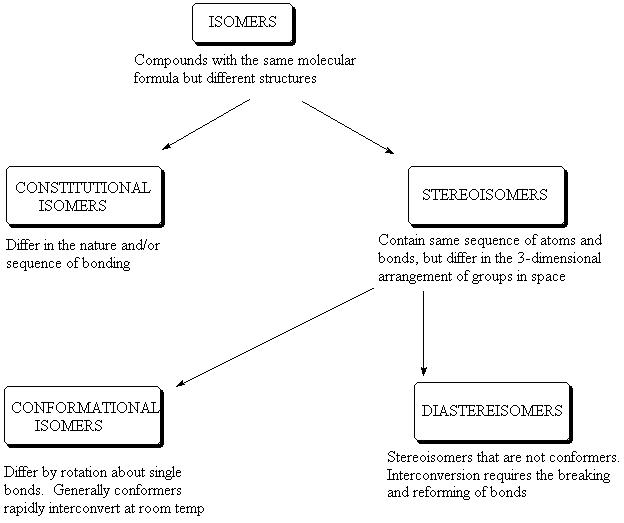
Examples Of Conformational Isomers. Third the number of electrons in the pi system must be 2 6 10 14 18 or a higher. Aromatic vs Antiaromatic vs Non Aromatic Practice Exercises. This type of stereoisomerism can be made possible by interconverting chemical bonds without breaking them. The compound having same molecular formula but differ in properties are known as isomers and the phenomenon is known as isomerism.
 Difference Between Configurational And Conformational Isomers Definition Structure Examples From pediaa.com
Difference Between Configurational And Conformational Isomers Definition Structure Examples From pediaa.com
Learn more about isomerism in this article. Boiling points are a measure of intermolecular forces. Third the number of electrons in the pi system must be 2 6 10 14 18 or a higher. The two propanol isomers consist of propan-1-ol and. Examples of Weak Acids Weak Acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions. Wikimedia commons by MrHolmium 2.
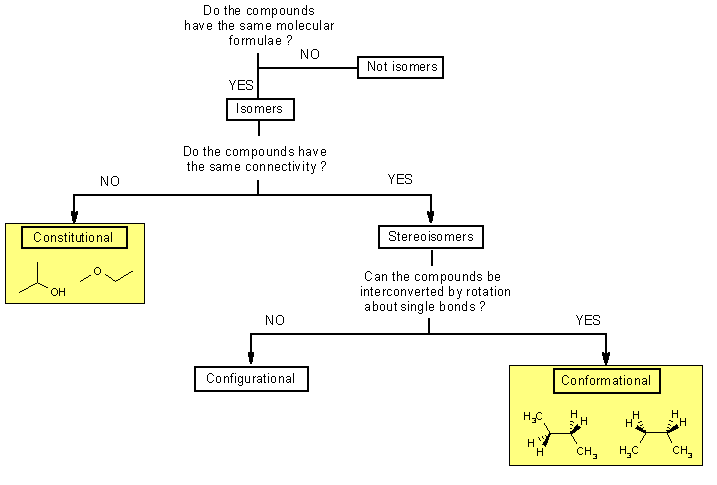
The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond.
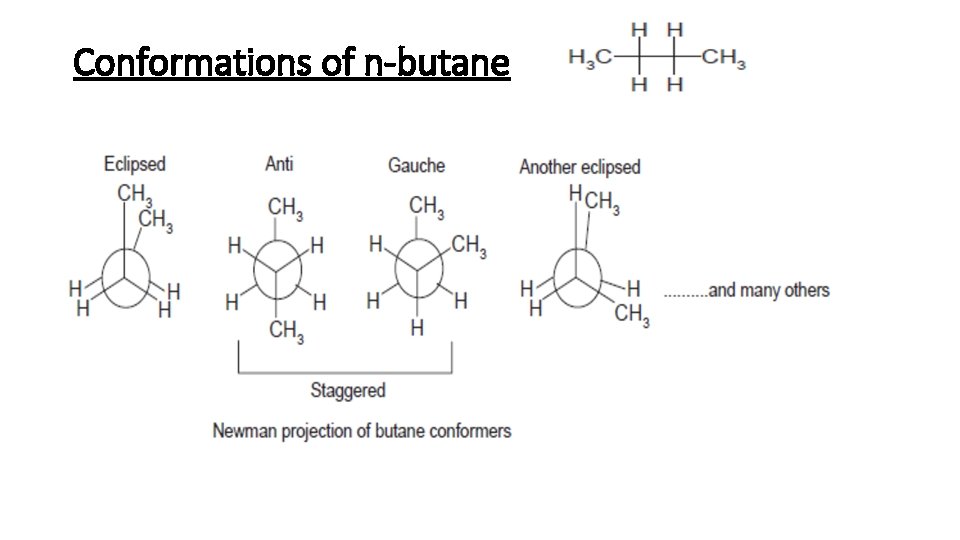
General formula for alkanes is C n H 2n2All carbon atoms tend to complete their tetra valency by bonding with the same or different atoms. Conformational isomers that are isolable due to high energy barriers are called atropisomers. The strength of the four main intermolecular forces and therefore their impact on boiling points is ionic hydrogen bonding dipole dipole dispersion Boiling point increases with molecular weight. The xanthine oxidase inhibitor allopurinol used. The therapeutic effects of some drugs are a direct consequence of enzyme inhibition eg. Conformational isomers exist in a dynamic equilibrium where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers.
 Source: swiflearn.com
Source: swiflearn.com
From understanding geometric isomers to naming molecules with Cis Trans or EZ and lots of practice examples to ensure the concept. The compound having same molecular formula but differ in properties are known as isomers and the phenomenon is known as isomerism. General formula for alkanes is C n H 2n2All carbon atoms tend to complete their tetra valency by bonding with the same or different atoms. This results in conformational changes hence disrupting the enzyme activity. The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
Isomers of Butane Constitutional Isomers of Butane Conformational Isomers of Butane. The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond. The two propanol isomers consist of propan-1-ol and. Compounds B and C provide. Examples of drugs that inhibit the metabolism of the active S and less active R isomers of warfarin in this way are shown in Table 106.
 Source: differencebetween.net
Source: differencebetween.net
If we monitor the ball and stick model of ethane and rotate one carbon atom keeping another carbon atom still around the C-C axis. Which of the following statements concerning conformations is. The therapeutic effects of some drugs are a direct consequence of enzyme inhibition eg. The intermolecular forces increase with increasing polarization ie. Examples of drugs that inhibit the metabolism of the active S and less active R isomers of warfarin in this way are shown in Table 106.
 Source: pediaa.com
Source: pediaa.com
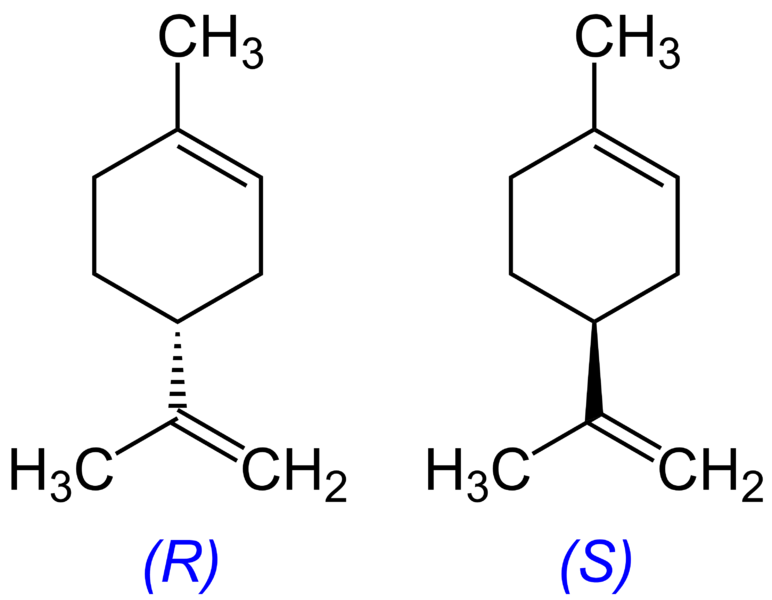
Isomers which are non-superimposable mirror images of each other are known as enantiomers. Isomers of Butane Constitutional Isomers of Butane Conformational Isomers of Butane. In conformational isomerism because of the free rotation of carbon-carbon single bond different arrangement of atoms in space are obtained. A structural isomers b geometric isomers c conformational structures d identical structures e optical isomers 13. This tutorial will give you a detailed understand of when and how to use each of these isomers in your organic chemistry course.
 Source: sciencedirect.com
Source: sciencedirect.com
Conformational isomers that are isolable due to high energy barriers are called atropisomers. The two propanol isomers consist of propan-1-ol and. They can not be separated. A structural isomers b geometric isomers c conformational structures d identical structures e optical isomers 13. A shotgun lipidomics approach enabled by photochemical reaction.
 Source: sciencedirect.com
Source: sciencedirect.com
Difference in electronegativity of bonds. What are they whats the difference and when to use each case. Compounds B and C provide. If we monitor the ball and stick model of ethane and rotate one carbon atom keeping another carbon atom still around the C-C axis. The xanthine oxidase inhibitor allopurinol used.
 Source: sydney.edu.au
Source: sydney.edu.au
The chemical structure C 3 H 8 O exists as several isomers of propanol as well as the isomer methoxyethane. Cis vs Trans Isomers and E vs Z Isomers. General formula for alkanes is C n H 2n2All carbon atoms tend to complete their tetra valency by bonding with the same or different atoms. Isomers which are non-superimposable mirror images of each other are known as enantiomers. Example of an Isomer.
 Source: slideplayer.com
Source: slideplayer.com
Lets understand the fundamentals of conformation with the examples of ethane. Isomerism the existence of molecules that have the same numbers of the same kinds of atoms and hence the same formula but differ in chemical and physical properties. Isomers are especially important in nutrition and medicine because enzymes tend to work on one isomer over another. Theobromine caffeine and theophylline are isomers differing in the placement of methyl groups. Where K is the equilibrium constant ΔG is the difference in standard free energy between the two conformers in kcalmol R is the universal.
 Source: researchgate.net
Source: researchgate.net
How many alcohols are structural isomers with the formula. Isomerism the existence of molecules that have the same numbers of the same kinds of atoms and hence the same formula but differ in chemical and physical properties. Learn more about isomerism in this article. Third the number of electrons in the pi system must be 2 6 10 14 18 or a higher. Examples of Weak Acids Weak Acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions.
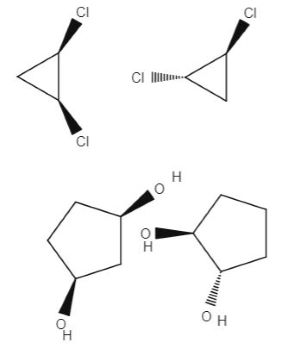 Source: study.com
Source: study.com
A cyclic compound or ring compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ringRings may vary in size from three to many atoms and include examples where all the atoms are carbon ie are carbocycles none of the atoms are carbon inorganic cyclic compounds or where both. Cis vs Trans Isomers and E vs Z Isomers. Aromatic vs Antiaromatic vs Non Aromatic Practice Exercises. Examples of drugs that inhibit the metabolism of the active S and less active R isomers of warfarin in this way are shown in Table 106. The therapeutic effects of some drugs are a direct consequence of enzyme inhibition eg.
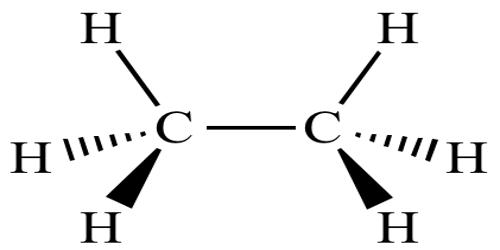 Source: assignmentpoint.com
Source: assignmentpoint.com
How many alcohols are structural isomers with the formula. The chemical structure C 3 H 8 O exists as several isomers of propanol as well as the isomer methoxyethane. There are several examples of isomers described as follows. Theobromine caffeine and theophylline are isomers differing in the placement of methyl groups. B Conformational isomerism.
 Source: slideshare.net
Source: slideshare.net
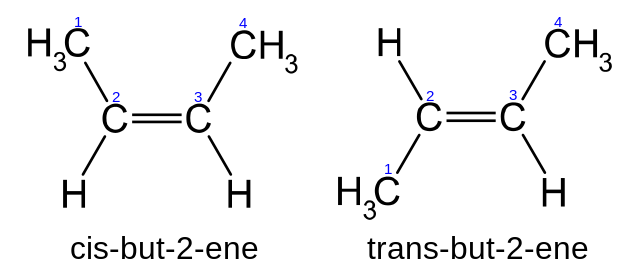
When the group of atoms that make up the molecules of different isomers are bonded together in fundamentally different ways we refer to such compounds as constitutional isomersFor example in the case of the C 4 H 8 hydrocarbons. A 5 b 6 c 7 d 8 e 9 12. Conformational isomerism is a diastereomerism where two isomers differ because of the rotation around the carbon-carbon single bond. Learn more about isomerism in this article. C 5 H 11 OH.
 Source: pediaa.com
Source: pediaa.com
A shotgun lipidomics approach enabled by photochemical reaction. First it must be cyclic Second every atom around the ring must have an available p-orbital. The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond. Examples of drugs that inhibit the metabolism of the active S and less active R isomers of warfarin in this way are shown in Table 106. Theobromine caffeine and theophylline are isomers differing in the placement of methyl groups.

The therapeutic effects of some drugs are a direct consequence of enzyme inhibition eg. What are they whats the difference and when to use each case. As defined in an earlier introductory section isomers are different compounds that have the same molecular formula. Example of an Isomer. Examples of Conformational Isomers.
 Source: pediaa.com
Source: pediaa.com
The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond. Conformational isomers that are isolable due to high energy barriers are called atropisomers. Wikimedia commons by MrHolmium 2. The two propanol isomers consist of propan-1-ol and. Alkanes are the simplest hydrocarbons with all C-C bonds.
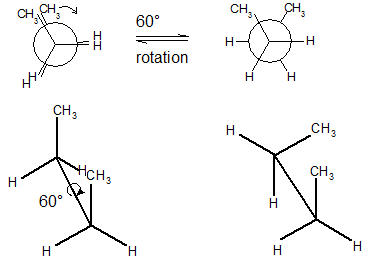 Source: study.com
Source: study.com
During the loose conformational change ADP and inorganic phosphate Pi substrates are allowed to bind together loosely but the reaction that synthesizes ATP is not catalyzed until the tight change. Contrast Examples 625. Isomers which are non-superimposable mirror images of each other are known as enantiomers. Isomerism the existence of molecules that have the same numbers of the same kinds of atoms and hence the same formula but differ in chemical and physical properties. The xanthine oxidase inhibitor allopurinol used.
 Source: slidetodoc.com
Source: slidetodoc.com
If we monitor the ball and stick model of ethane and rotate one carbon atom keeping another carbon atom still around the C-C axis. Isomers which are non-superimposable mirror images of each other are known as enantiomers. The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond. Such isomers are called stereoisomers from the Greek stereos meaning solid. This tutorial will give you a detailed understand of when and how to use each of these isomers in your organic chemistry course.
 Source: chem.ucalgary.ca
Source: chem.ucalgary.ca
General formula for alkanes is C n H 2n2All carbon atoms tend to complete their tetra valency by bonding with the same or different atoms. During the loose conformational change ADP and inorganic phosphate Pi substrates are allowed to bind together loosely but the reaction that synthesizes ATP is not catalyzed until the tight change. How many alcohols are structural isomers with the formula. Isomers are especially important in nutrition and medicine because enzymes tend to work on one isomer over another. If we monitor the ball and stick model of ethane and rotate one carbon atom keeping another carbon atom still around the C-C axis.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title examples of conformational isomers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






