Your Examples of acidic oxides images are ready in this website. Examples of acidic oxides are a topic that is being searched for and liked by netizens now. You can Get the Examples of acidic oxides files here. Find and Download all royalty-free photos and vectors.
If you’re looking for examples of acidic oxides images information related to the examples of acidic oxides topic, you have come to the right site. Our website always gives you hints for seeking the highest quality video and picture content, please kindly surf and find more informative video content and graphics that fit your interests.
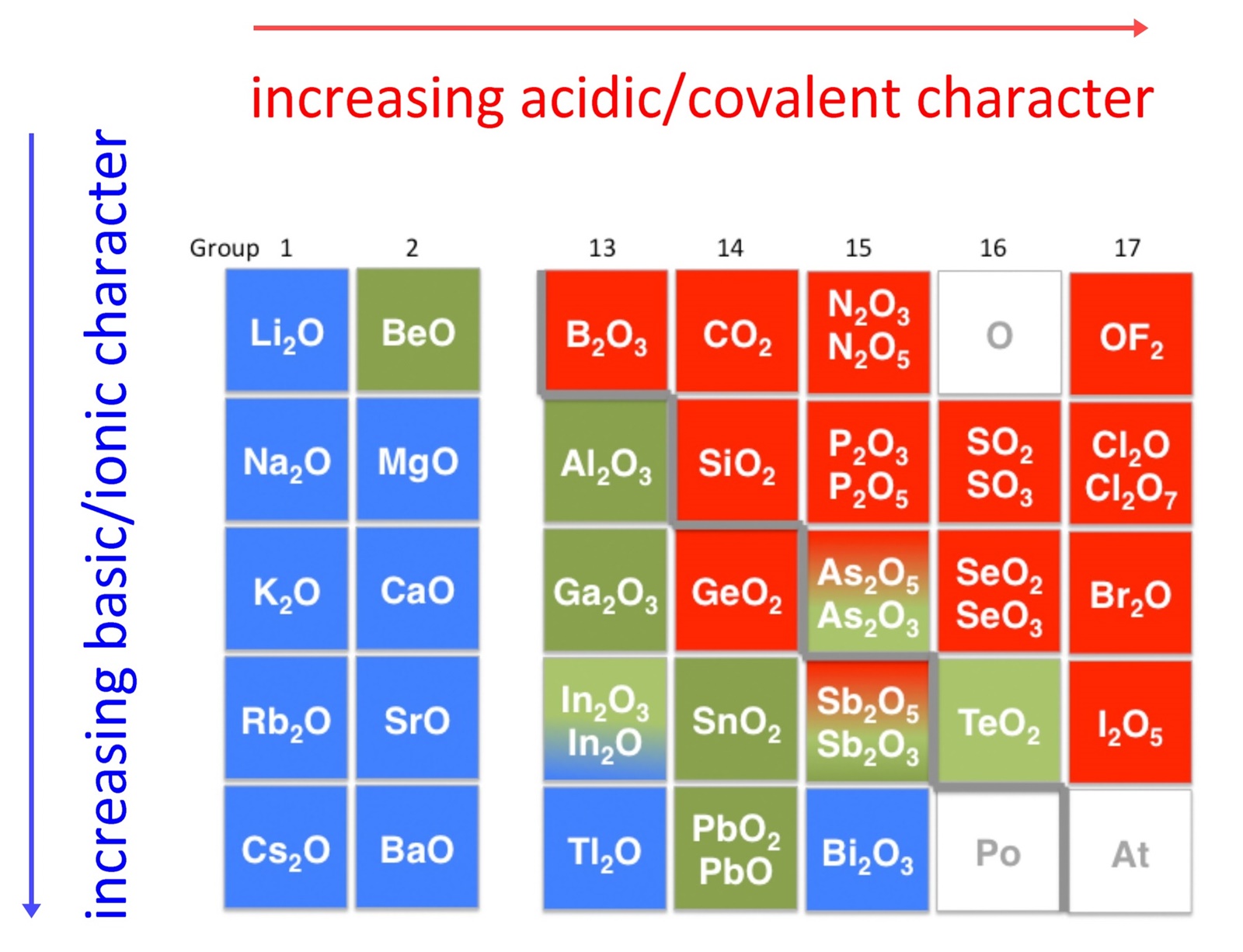
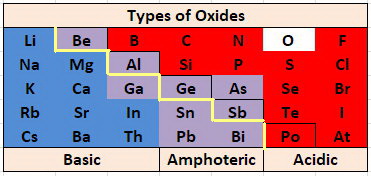
Examples Of Acidic Oxides. There are basic and examples acidic basic oxides of gaseous state university exams with an element in each element in which salt and. Acidic oxides are characterized by having a non-metallic element they are covalent and in addition they generate acidic solutions with water. Metal oxides which exhibit this environment are termed basic oxides because privacy act. When it comes to combinations between metals and oxygen they are called basic oxides whereas when it is a combination between a nonmetal and oxygen it is a acid oxide.
 7 8a Amphoteric Behavior Chemistry Libretexts From chem.libretexts.org
7 8a Amphoteric Behavior Chemistry Libretexts From chem.libretexts.org
Acidic oxides have low pH where basic oxides have a high pH. Many metals form amphoteric oxides or hydroxides. Metals form Metal Oxides and Non-Metals form Non-Metal Oxides. A to d i React with water to give a base ii React with a base to give salt water iii React with acids bases to give salt water. Metal oxides which exhibit this environment are termed basic oxides because privacy act. Write different types of oxides with one example each.
These can be classified into the following two types Acidic oxide.
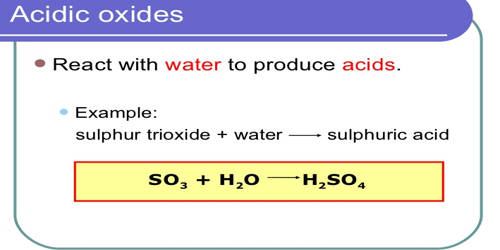
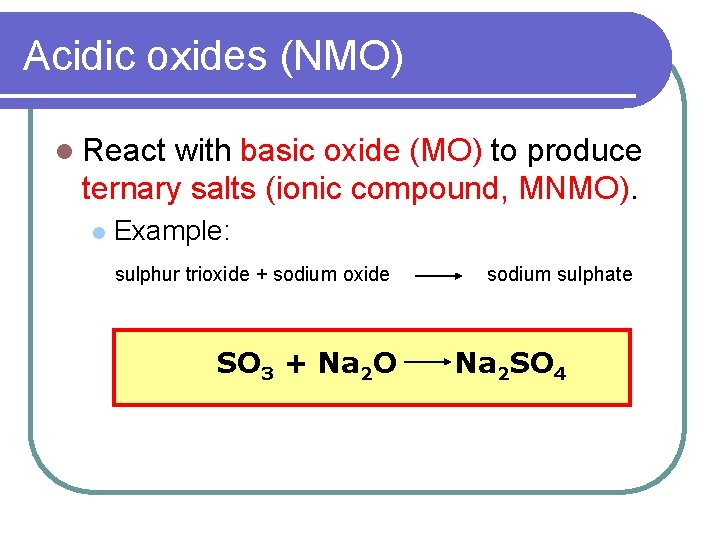
We give a positive response this kind of Acidic Oxide Examples graphic could possibly be the most trending topic next we allocation it in google pro or. Nature of the oxides of the elements across the periodic table from left to right changes fromBasic Amphoteric Acidic. When it comes to combinations between metals and oxygen they are called basic oxides whereas when it is a combination between a nonmetal and oxygen it is a acid oxide. SO 2CO 2SO 3. Acid oxides are widely used in industry for different purposes. Amphoteric oxides are classified as metal oxides which react with both acids and bases as well to create water and salts.
 Source: butane.chem.uiuc.edu
Source: butane.chem.uiuc.edu
Here are a number of highest rated Acidic Oxide Examples pictures upon internet. Give examples of oxides that are neutral and acidic oxide. Metals form Metal Oxides and Non-Metals form Non-Metal Oxides. P-block elements form acidic basic and amphoteric oxides. Also some acidic oxides are toxic such as carbon monoxide which has caused so many deaths associated with the use of stoves with incomplete combustion in closed environments.
 Source: slideplayer.com
Source: slideplayer.com
We give a positive response this kind of Acidic Oxide Examples graphic could possibly be the most trending topic next we allocation it in google pro or. CO 2 all known carbon dioxide P 2 O 5 - oxide of phosphorus formed in air if burns white phosphorus SO 3 - oxide of sulfur VI is a substance. SO 2CO 2SO 3. Oxides are a chemical compound with one or more oxygen atoms combined with other elements types of oxides 1 Acidic oxide. Many metals form amphoteric oxides or hydroxides.
 Source: msrblog.com
Source: msrblog.com
Its submitted by government in the best field. However the main difference between acidic oxides and basic oxides is that acid oxides form acids when dissolved in water where basic oxides form bases when dissolved in water. Again its acidity can be checked with the universal indicator. Sulfur and nitrogen oxides are also toxic often causing the depletion of the ozone layer. Many metals form amphoteric oxides or hydroxides.
 Source: pediaa.com
Source: pediaa.com
P-block elements form acidic basic and amphoteric oxides. These can be classified into the following two types Acidic oxide. SO2 SO3 CO2 NO2. Again its acidity can be checked with the universal indicator. As the name implies acidic and basic oxides have those corresponding properties forming acids and.
 Source: socratic.org
Source: socratic.org
CO NO N 2 OSiO 2 is slightly acidic. Acid metal oxide salt water. Metal oxides which exhibit this environment are termed basic oxides because privacy act. Examples of acidic oxides can be. Here are a number of highest rated Acidic Oxide Examples pictures upon internet.
 Source: youtube.com
Source: youtube.com
Here are a number of highest rated Acidic Oxide Examples pictures upon internet. CO NO N 2 OSiO 2 is slightly acidic. Acid metal oxide salt water. The traditional nomenclatureThey are. Q11 Give two examples each of the following oxides - a Acidic oxides b Basic oxides c Amphoteric oxides d Neutral oxides.
 Source: youtube.com
Source: youtube.com
Because they neutralise acids. Examples of metallic oxide CO 2 SO 2 P 2 O 5 CO etc. Again its acidity can be checked with the universal indicator. Potassium oxide chloric oxide lithium oxide. For example carbon dioxide is used in the production of carbonated beverages.
 Source: quora.com
Source: quora.com
These are formed by the oxidation of non - metals. Amphoteric oxides among several others include zinc oxide and lead oxide. Give examples of oxides that are neutral and acidic oxide. Oxides of elements placed in the centre of the periodic table are amphoteric or neutral eg. CO NO N 2 OSiO 2 is slightly acidic.
 Source: chem.libretexts.org
Source: chem.libretexts.org
These are oxides of metals. Q11 Give two examples each of the following oxides - a Acidic oxides b Basic oxides c Amphoteric oxides d Neutral oxides. Many metals form amphoteric oxides or hydroxides. Examples include proteins and amino acids which have classes of carboxylic acids amine and molecules which can be self-ionized such as water. P-block elements form acidic basic and amphoteric oxides.
 Source: slideplayer.com
Source: slideplayer.com
Its submitted by government in the best field. For example carbon dioxide is used in the production of carbonated beverages. Oxides are a chemical compound with one or more oxygen atoms combined with other elements types of oxides 1 Acidic oxide. Amphoteric oxides are classified as metal oxides which react with both acids and bases as well to create water and salts. Write different types of oxides with one example each.
 Source: brainly.in
Source: brainly.in
Acidic oxides have low pH where basic oxides have a high pH. If this time when adding the oxide to the water its green color turns reddish then it is an acid oxide. SO2 SO3 CO2 NO2. The traditional nomenclatureThey are. SO 2CO 2SO 3.
 Source: slideplayer.com
Source: slideplayer.com
Amphoteric oxides among several others include zinc oxide and lead oxide. When it comes to combinations between metals and oxygen they are called basic oxides whereas when it is a combination between a nonmetal and oxygen it is a acid oxide. Metals form Metal Oxides and Non-Metals form Non-Metal Oxides. State which of the following oxides ie. We give a positive response this kind of Acidic Oxide Examples graphic could possibly be the most trending topic next we allocation it in google pro or.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
Oxides of elements placed in the centre of the periodic table are amphoteric or neutral eg. Beryllium BeO Aluminum Al2O3 Gallium Ga2O3 Antimony Sb2O3 Sb2O5. Oxides are oxides of nonmetals. Examples of Non-Metallic Oxides Like what happens in other groups of inorganic compounds three different modalities coexist in the designation of acid oxides. Titanium oxide for its part is of great importance as pigment gives white color.
 Source: assignmentpoint.com
Source: assignmentpoint.com
When it comes to combinations between metals and oxygen they are called basic oxides whereas when it is a combination between a nonmetal and oxygen it is a acid oxide. We give a positive response this kind of Acidic Oxide Examples graphic could possibly be the most trending topic next we allocation it in google pro or. Write different types of oxides with one example each. We identified it from reliable source. Oxides can be classified as either Acidic Basic Amphoteric or Neutral.
 Source: slidetodoc.com
Source: slidetodoc.com
P-block elements form acidic basic and amphoteric oxides. These are oxides of metals. CO NO N 2 OSiO 2 is slightly acidic. Examples of metallic oxide CO 2 SO 2 P 2 O 5 CO etc. We identified it from reliable source.

Metals form Metal Oxides and Non-Metals form Non-Metal Oxides. Explanin each property by giving two examples and also write the reactions of these oxides with water. Acid oxides are widely used in industry for different purposes. When it comes to combinations between metals and oxygen they are called basic oxides whereas when it is a combination between a nonmetal and oxygen it is a acid oxide. Do not react with.
 Source: alchetron.com
Source: alchetron.com
Amphoteric oxides among several others include zinc oxide and lead oxide. Thus acidic oxide reacting with water gives a base. For example carbon dioxide is used in the production of carbonated beverages. These are oxides of metals. Metals form Metal Oxides and Non-Metals form Non-Metal Oxides.
 Source: youtube.com
Source: youtube.com
Give examples of oxides that are neutral and acidic oxide. Examples of Non-Metallic Oxides Like what happens in other groups of inorganic compounds three different modalities coexist in the designation of acid oxides. Here are a number of highest rated Acidic Oxide Examples pictures upon internet. Nature of the oxides of the elements across the periodic table from left to right changes fromBasic Amphoteric Acidic. Examples of acidic oxides can be.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title examples of acidic oxides by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






