Your Example of thermal decomposition reaction images are available in this site. Example of thermal decomposition reaction are a topic that is being searched for and liked by netizens now. You can Get the Example of thermal decomposition reaction files here. Download all royalty-free images.
If you’re searching for example of thermal decomposition reaction pictures information related to the example of thermal decomposition reaction keyword, you have visit the right site. Our site frequently gives you hints for viewing the highest quality video and image content, please kindly hunt and find more informative video articles and graphics that fit your interests.
Example Of Thermal Decomposition Reaction. Examples of Thermal decomposition reactions. A common example of a thermal decomposition reaction is provided below. It requires energy to break the bonds between reactants thus is an endothermic process. Ii Thermal decomposition reactions form one of the steps in extraction of metals.
 What Is Thermal Decomposition Reaction Edurev Class 10 Question From edurev.in
What Is Thermal Decomposition Reaction Edurev Class 10 Question From edurev.in
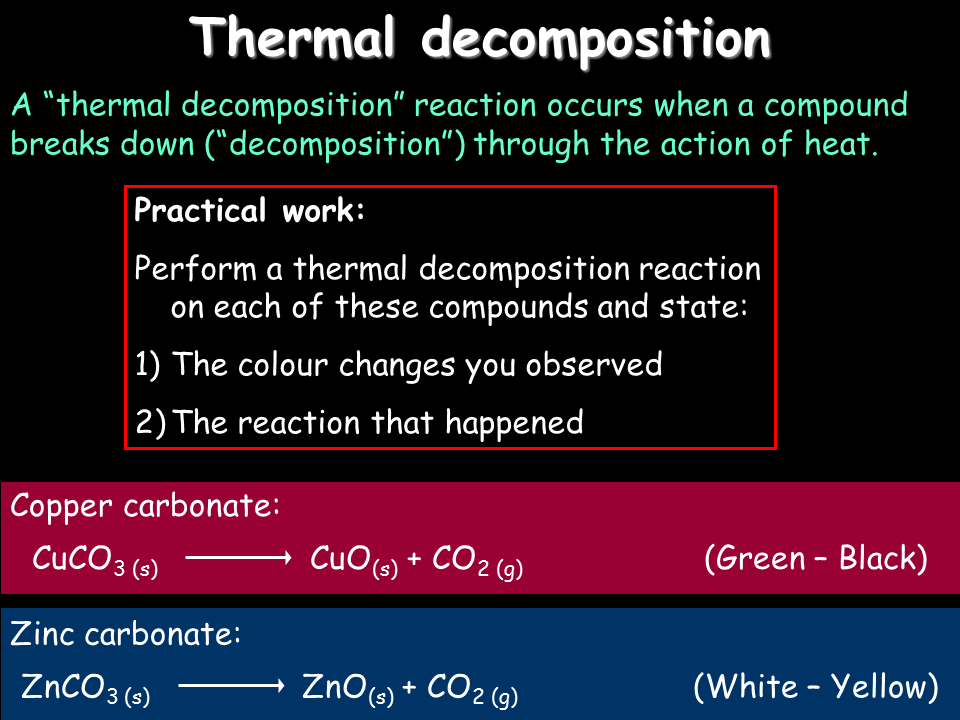
CaCO3 CaO CO2 When heated calcium carbonate decomposes into calcium oxide and carbon dioxide. AB - A B. The 4 examples above are decomposition due to heating. This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source. Carbonates decompose into carbon dioxide and an oxide. Was this answer helpful.
This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source.
Medium Solution Verified by Toppr Thermal decomposition reactions are those reactions which break up or decompose on heating to form many products. This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source. Thermal decomposition reactions are common in everyday life. Heating magnesium carbonate to get magnesium oxide and carbon dioxide. Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce. An example of t Continue Reading Related Answer Aman Sharma former Student.
 Source: youtube.com
Source: youtube.com
These reactions are the endothermic type of reaction as it requires external heat for reaction. This is represented by a general formula format. 2Fe OH 3 heatFe 2 O 3 3H 2 O Ques. Thermal decomposition reactions refer to the chemical reaction which decomposes on heating to form many products. For example Zinc carbonate the naturally occurring ore of zinc is first decomposed to give zinc oxide and then reduced to obtain zinc metal ie Decomposition Reactions in our body.
 Source: brainly.in
Source: brainly.in
ZnCO3 oversetheatrightarrow. Thermal decomposition reaction is also known as thermolysis. The examples of thermal decomposition include. 2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. For example Zinc carbonate the naturally occurring ore of zinc is first decomposed to give zinc oxide and then reduced to obtain zinc metal ie Decomposition Reactions in our body.
Thermal composition reaction or thermolysis is the decomposition by means of heat. Explain with an example. Give an example each for thermal decomposition and photochemical decomposition reactions. What are the unique characteristics of a decomposition reaction. Examples of Thermal decomposition reactions.
 Source: drgpinstitute.in
Source: drgpinstitute.in
CuSO4 CuO SO3 H2O SO3 H2SO4. The digestion of food in the body is an example of decomposition reaction. The decomposition of potassium chlorate to form potassium chloride and oxygen gas when hated 2KClO 3 sheat2KCl s3O 2 g The decomposition of ferric dioxide into ferric oxide and water on heating. 2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. ZnCO3 oversetheatrightarrow.
 Source: slideplayer.com
Source: slideplayer.com
The 4 examples above are decomposition due to heating. Thermal decomposition is represented by a general formula format. Share It On Facebook Twitter Email. ZnCO3 oversetheatrightarrow. What are the unique characteristics of a decomposition reaction.
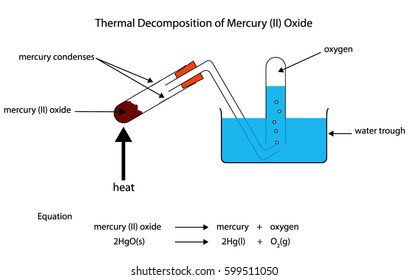 Source: shutterstock.com
Source: shutterstock.com
2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. The digestion of food in the body is an example of decomposition reaction. Examples of thermal dissociation are. There are three types of decomposition reactions. A simple example of decomposition reaction is hydrolysis of water where a water molecule is broken down into hydrogen and oxygen gas.
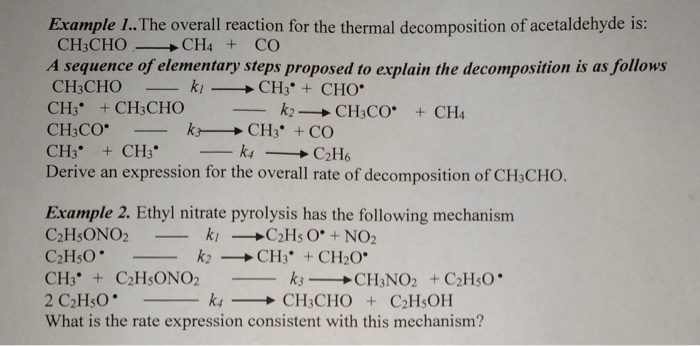
Answered Feb 7 2018 by Md samim 952k points selected Feb 7 2018 by sforrest072. A simple example of decomposition reaction is hydrolysis of water where a water molecule is broken down into hydrogen and oxygen gas. The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction. It requires energy to break the bonds between reactants thus is an endothermic process. Thermal decomposition reactions refer to the chemical reaction which decomposes on heating to form many products.

The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction. A solid remains in the tube but its identity is now copper oxide CuO. What are the unique characteristics of a decomposition reaction. Decomposition reaction examples in real life Decomposition reactions are chemical reactions in which a more complex molecule breaks down to make simpler ones. Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce.
 Source: toppr.com
Source: toppr.com
CaCO3 CaO CO2 When heated calcium carbonate decomposes into calcium oxide and carbon dioxide. Explain with an example. What are the unique characteristics of a decomposition reaction. Zinc carbonate on heating decomposes to form Zinc oxide and carbon dioxide. Was this answer helpful.
 Source: icsehelp.com
Source: icsehelp.com
In a decomposition reaction a compound is broken into smaller chemical species. The equation is represented below. A solid remains in the tube but its identity is now copper oxide CuO. Was this answer helpful. Metal carbonates thermally decompose into metal oxides and carbon dioxide gas.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction. If a test tube of CuCO 3 is heated in a Bunsen burner the solid powder jumps around as molecules of carbon dioxide gas are released. Heating magnesium carbonate to get magnesium oxide and carbon dioxide. Zinc carbonate on heating decomposes to form Zinc oxide and carbon dioxide. H₂O₂ H₂ O₂ Classification of Decomposition Reaction.
 Source: learneasytutorial.com
Source: learneasytutorial.com
The presence of carbon dioxide gas can be tested using limewater. This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source. 2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. What is the decomposition reaction of barium carbonate. The process is not reversible.
 Source: edurev.in
Source: edurev.in
A simple example of decomposition reaction is hydrolysis of water where a water molecule is broken down into hydrogen and oxygen gas. Answered Feb 7 2018 by Md samim 952k points selected Feb 7 2018 by sforrest072. Thermal composition reaction or thermolysis is the decomposition by means of heat. Give an example each for thermal decomposition and photochemical decomposition reactions. Hence they are all examples of Thermal Decomposition Reaction.
 Source: researchgate.net
Source: researchgate.net
2Fe OH 3 heatFe 2 O 3 3H 2 O Ques. A great example of a thermal decomposition reaction which is the type of decomposition that occurs with the addition of heat is the breakdown of copper carbonate CuCO 3. Thermal decomposition is represented by a general formula format. Thermal decomposition of copper sulphate When you put a little amount of blue copper sulphate in a clean test tube then heat the content of the test by using a flame A black substance will be formed Blue copper sulphate decomposes by the heat into copper oxide black colour and sulphur trioxide. Thermal decomposition reactions refer to the chemical reaction which decomposes on heating to form many products.
 Source: brainly.in
Source: brainly.in
The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction. Heating magnesium carbonate to get magnesium oxide and carbon dioxide. When we eat foods like wheat rice. When performing a thermal decomposition reaction it is important to avoid suck-back at the end of the experiment. Calcium carbonate is heated to decompose into calcium oxide and carbon dioxide.
 Source: edurev.in
Source: edurev.in
Calcium carbonate is heated to decompose into calcium oxide and carbon dioxide. AB - A B. The decomposition of potassium chlorate to form potassium chloride and oxygen gas when hated 2KClO 3 sheat2KCl s3O 2 g The decomposition of ferric dioxide into ferric oxide and water on heating. 2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. 2Fe OH 3 heatFe 2 O 3 3H 2 O Ques.
 Source: brainly.in
Source: brainly.in
A great example of a thermal decomposition reaction which is the type of decomposition that occurs with the addition of heat is the breakdown of copper carbonate CuCO 3. Thermal decomposition is represented by a general formula format. Examples of thermal dissociation are. A thermal decomposition reaction occurs when heat is applied to a compound causing it to decompose break down into multiple different chemical substances. There are three types of decomposition reactions.
 Source: brainly.in
Source: brainly.in
A simple example of decomposition reaction is hydrolysis of water where a water molecule is broken down into hydrogen and oxygen gas. What are the uses of decomposition reactions. It requires energy to break the bonds between reactants thus is an endothermic process. A common example of a thermal decomposition reaction is provided below. Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title example of thermal decomposition reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






