Your Endothermic reaction examples equations images are ready in this website. Endothermic reaction examples equations are a topic that is being searched for and liked by netizens today. You can Get the Endothermic reaction examples equations files here. Download all free photos.
If you’re looking for endothermic reaction examples equations images information related to the endothermic reaction examples equations topic, you have pay a visit to the right blog. Our website frequently gives you suggestions for downloading the highest quality video and image content, please kindly search and locate more informative video content and images that match your interests.
Endothermic Reaction Examples Equations. Endothermic Reaction Definition. The reaction requires the addition of energy to the reactants to form the bonds in the products. The energy change that accompanies a reaction can be written in the chemical equation. Combustion is an example of an exothermic reaction.
 Cbse Class 10 Learn About Exothermic And Endothermic Reaction And Some Question In Hindi Offered By Unacademy From unacademy.com
Cbse Class 10 Learn About Exothermic And Endothermic Reaction And Some Question In Hindi Offered By Unacademy From unacademy.com
The heat energy breaks the bonds in the substance causing the reaction. The reaction between barium hydroxide and ammonium chloride the reaction between ethanoic acid and sodium carbonate Exothermic and endothermic reactions are used extensively in everyday life and in industry. Reactants Energy Products. Reactants Energy Products. A good example of an endothermic reaction is photosynthesis. The chemical equation can be written as follows.
Clouds come into existence from condensation of water vapor.
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Characteristics equations and examples A endothermic reaction It i one that to take place mut aborb energy in the form of heat or radiation from it urrounding. Endothermic Reaction Examples. SO S O ΔH 297 KJ exothermic reaction. These reactions cause a cooling effect by lowering the temperature of the surrounding environment. This is actually one of the key characteristics of an endothermic reaction.
 Source: slideplayer.com
Source: slideplayer.com
Write Equation Here Lead a discussion on some common exothermic and endothermic reactions. An endothermic reaction uses energy as a reactant. The energy change that accompanies a reaction can be written in the chemical equation. The heat energy breaks the bonds in the substance causing the reaction. Students measure the temperature changes in different reactions taking place in a polystyrene cup classifying the reactions as exothermic or endothermic.
 Source: drgpinstitute.in
Source: drgpinstitute.in
Below are some endothermic reaction examples in everyday life and example equations. NH4Cl s H2O l NH4Cl aq - Heat 10 examples of. The chemical equation can be written as follows. Fusion of snow on a warm windshield especially for heavy snow-wet. Endothermic ExamplesEndothermic Reaction Examples Equations.
 Source: slidetodoc.com
Source: slidetodoc.com
Endothermic Reaction Examples. SO S O ΔH 297 KJ exothermic reaction. Chemical reactions are all about the energy. Discuss demonstration write the chemical reaction on the board. Some examples of endothermic reactions are.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Reactants Energy Products. In this case the chemical equations for endothermic and exothermic reactions would be as follows. The sign for the energy change is. Endothermic reactions have positive enthalpy values ΔH. Endothermic changes are very common in nature.
 Source: youtube.com
Source: youtube.com
In an exothermic reaction change in enthalpy ΔH will be negative. The reaction between barium hydroxide and ammonium chloride the reaction between ethanoic acid and sodium carbonate Exothermic and endothermic reactions are used extensively in everyday life and in industry. The heat energy breaks the bonds in the substance causing the reaction. Endothermic and exothermic reactions are chemical reactions that absorb and release heat respectively. Airbags a safety device in modern cars utilize an exothermic reaction.
 Source: toppr.com
Source: toppr.com
Clouds come into existence from condensation of water vapor. Heat energy is absorbed from the pan to cook the egg. Mixture of cement and water exothermic Melting and freezing of ice endothermic and exothermic. The heat energy breaks the bonds in the substance causing the reaction. The salt dissociates into ammonium NH 4 and chloride Cl - ions.
 Source: icsehelp.com
Source: icsehelp.com
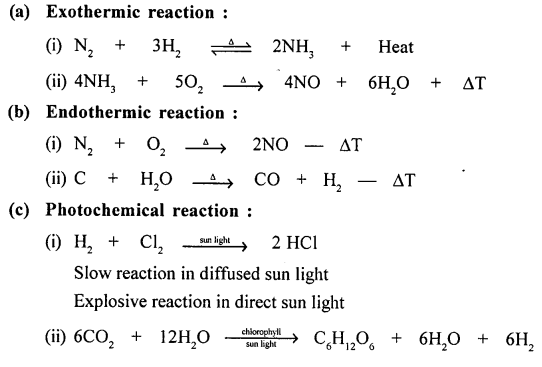
Making ice cube is a process of liquid changing its state to solid. Exothermic reaction lets out heat when the temperature of the surrounding objects goes on increasing. 2 Cooking an egg. A good example of an endothermic reaction is photosynthesis. Endothermic Reaction Examples When ammonium chloride NH 4 Cl is dissolved in water an endothermic reaction takes place 10 examples of endothermic reactions with equations.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Some examples of endothermic reactions are. In an exothermic reaction change in enthalpy ΔH will be negative. Making ice cube is a process of liquid changing its state to solid. Fusion of snow on a warm windshield especially for heavy snow-wet. NH4Cl s H2O l NH4Cl aq Heat.
 Source: slidetodoc.com
Source: slidetodoc.com
N 3H 2NH ΔH -92 KJ endothermic reaction. The reaction between barium hydroxide and ammonium chloride the reaction between ethanoic acid and sodium carbonate Exothermic and endothermic reactions are used extensively in everyday life and in industry. The sign for the energy change is. Fusion of snow on a warm windshield especially for heavy snow-wet. Photosynthesis is the chemical reaction that takes place in green plants which uses energy from the sun to change carbon dioxide and water into food that the plant needs to survive and which other organisms such as humans and other animals can eat so that they too can survive.
 Source: studiousguy.com
Source: studiousguy.com
Endothermic reactions are chemical processes in which the reactants absorb heat from the environment to be completed. Exothermic reactions have negative enthalpy values -ΔH. The natural gas methane burns in the air to form carbon dioxide and water and releases a large amount of heat. Reactants Energy Products. The drop in temperature may be great enough to cause liquids to freeze.
 Source: slideplayer.com
Source: slideplayer.com
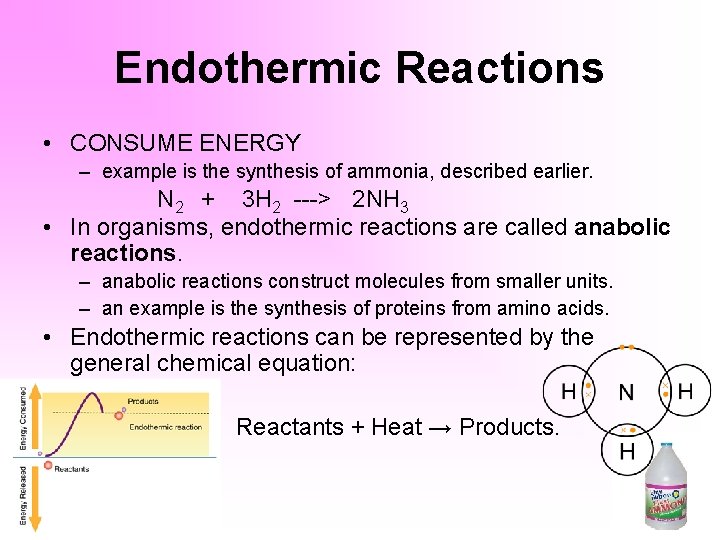
An endothermic reaction uses energy as a reactant. A good example of an endothermic reaction is photosynthesis. Reactants Energy Products. 2H2 O Heat 2H2O endothermic reaction Besides the term heat the heat changeenthalpy change can also be shown by a triangle ΔHsymbol. The drop in temperature may be great enough to cause liquids to freeze.
 Source: slideplayer.com
Source: slideplayer.com
In this reaction 43 kcal are needed to make the reaction occur. In an exothermic reaction change in enthalpy ΔH will be negative. The chemical equation can be written as follows. As the heat is absorbed the product will be colder. Endothermic changes are very common in nature.
 Source: khanacademy.org
Source: khanacademy.org
In endothermic reactions the temperature of the products is typically lower than the temperature of the reactants. These examples could be written as chemical reactions but are more generally considered to be endothermic or heat-absorbing processes. The energy change that accompanies a reaction can be written in the chemical equation. In this reaction 43 kcal are needed to make the reaction occur. Endothermic changes are very common in nature.
 Source: lorecentral.org
Source: lorecentral.org
Airbags a safety device in modern cars utilize an exothermic reaction. Snow formation in clouds is also an exothermic reaction. Generally but not alway they can be recognized by a drop in temperature in Content. The categorization of a reaction as endo- or exothermic depends on the net heat transfer. An exothermic reaction is a reaction in which energy is released in the form of light or heat.
 Source: khanacademy.org
Source: khanacademy.org
Endothermic changes are very common in nature. The experiments can also be used to revise different types of chemical reaction and with. Reactants Energy Products. Some examples of endothermic reactions are. As the heat is absorbed the product will be colder.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Reactants Energy Products. Fusion of snow on a warm windshield especially for heavy snow-wet. The chemical equation can be written as follows. Write Equation Here Lead a discussion on some common exothermic and endothermic reactions. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen.
 Source: toppr.com
Source: toppr.com
Endothermic reactions are chemical processes in which the reactants absorb heat from the environment to be completed. Energy Change in Endothermic Reactions The general equation for an endothermic reaction is. SO S O ΔH 297 KJ exothermic reaction. As the heat is absorbed the product will be colder. For example water absorbs heat from a burner to be converted into vapour.
 Source: lidolearning.com
Source: lidolearning.com
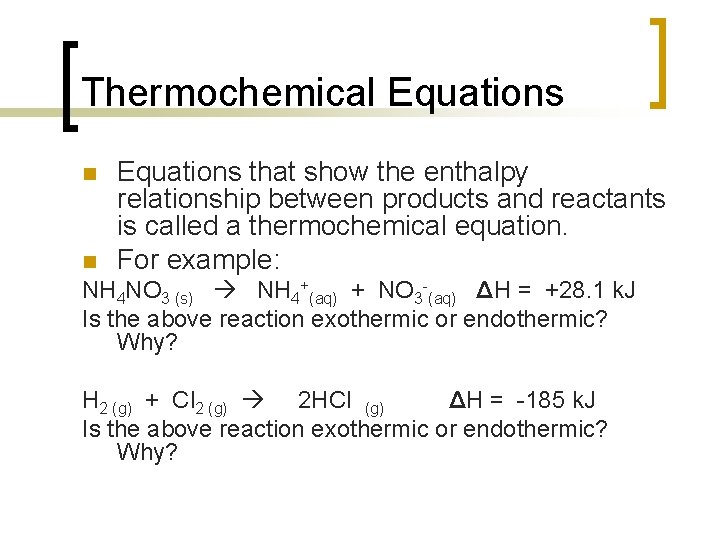
Thermochemical Equations See Moore 2 nd ed Sections 65 66 pp. Combustion is an example of an exothermic reaction. Heat energy is absorbed from the pan to cook the egg. Ammonium nitrate NH 4 NO 3 an important component in. In any given reaction heat is both.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title endothermic reaction examples equations by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






