Your Bronsted lowry acid examples images are available in this site. Bronsted lowry acid examples are a topic that is being searched for and liked by netizens now. You can Download the Bronsted lowry acid examples files here. Find and Download all free images.
If you’re searching for bronsted lowry acid examples images information related to the bronsted lowry acid examples interest, you have pay a visit to the ideal blog. Our website always provides you with suggestions for refferencing the maximum quality video and image content, please kindly surf and find more informative video articles and graphics that fit your interests.
Bronsted Lowry Acid Examples. Take the following reaction for example. A conjugated acid can donate a proton and base reforms. 15 How do Brønsted bases differ from Lewis bases explain with example. Label the Bronsted-Lowry acids A bases B conjugate acids CA and conjugate bases CB in the following reactions.
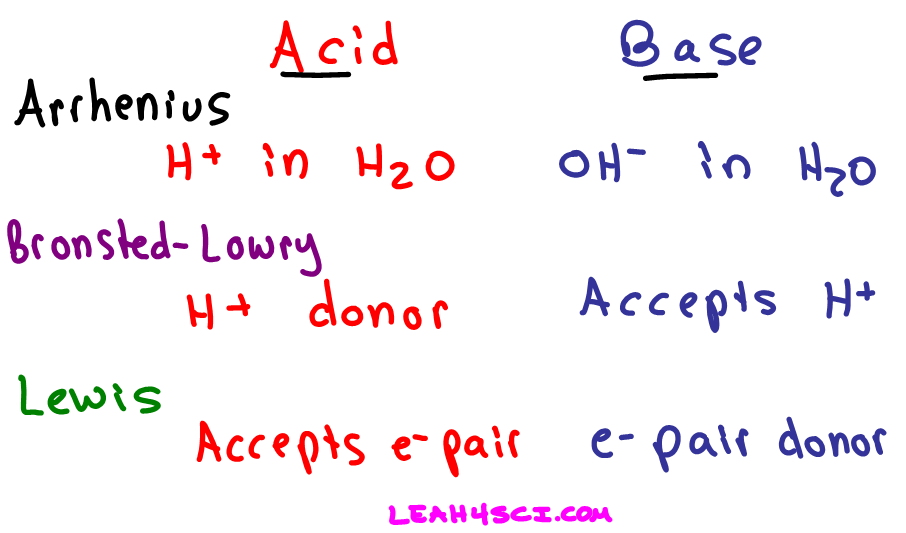 Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry From leah4sci.com
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry From leah4sci.com
Label the Bronsted-Lowry acids A bases B conjugate acids CA and conjugate bases CB in the following reactions. Ammonia is the Bronsted-Lowry base because it is the proton acceptor - it accepts a hydrogen atom from water. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton. Additionally because ammonia accepted the proton from hydrochloric acid the positive ammonia ion is the conjugate acid of the equation. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. The Bronsted-Lowry theory of an acid-base reaction involves the transfer of protons or H ions between the acid and base.
If given a balanced chemical equation eliminate the.
The following Bronsted-Lowry bases list is arranged in order of decreasing base strength. 11 Is NH3 acid or base. NH3 H2O NH4 OH-. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. Brønsted-Lowry Acids and Bases in Water. Ammonia is the Bronsted-Lowry base because it is the proton acceptor - it accepts a hydrogen atom from water.
 Source: chemistrylearner.com
Source: chemistrylearner.com
15 How do Brønsted bases differ from Lewis bases explain with example. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. 15 How do Brønsted bases differ from Lewis bases explain with example. A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction.

Bronsted-Lowry Base Examples. A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction. Acid-base reactions dont have to occur in water however. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton. Identify the Bronsted-Lowry acids and bases using the definitions.
 Source: slidetodoc.com
Source: slidetodoc.com
All strong acids behave the same in water – 1 M solutions of the strong acids all behave as 1 M solutions of the H 3 O ion – and very weak acids cannot act as acids in water. Since a Bronsted Lowry acid cannot occur without the presence of a Bronsted Lowry base the two are linked in what is called conjugate pairs. According to Bronsted-Lowry theory an acid is a proton H1 donor and a base is a proton acceptor. Brønsted Acids and Bases in Nonaqueous Solutions. 9 What is Lewis base with example.
 Source: chemistrylearner.com
Source: chemistrylearner.com
There are many compounds that can accept a pair of electrons from a Lewis base yet dont have any Brønsted-Lowry acid proton moiety. H 2 SO 4 - OH. According to the theory an acid and base react with each other causing the acid to form its conjugate base and the base to form its conjugate acid by exchanging a proton. For each example the following table will provide examples of equations and the acidbase pairs. The associated conjugate acid is.
 Source: youtube.com
Source: youtube.com
Identify these bases in the provided chemical equation examples demonstrating the exchange of ions. In contrast a Bronsted-Lowry base accepts hydrogen ions. There are many compounds that can accept a pair of electrons from a Lewis base yet dont have any Brønsted-Lowry acid proton moiety. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. If given a balanced chemical equation eliminate the.
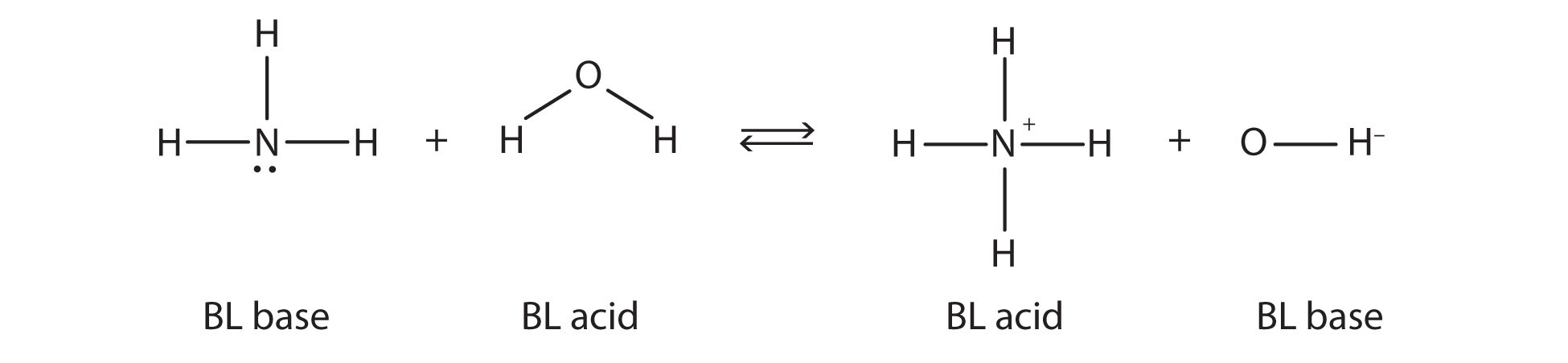 Source: chem.libretexts.org
Source: chem.libretexts.org
An example of a proton acceptor is ammonia NH3. - H 2 O - Cl1 OH1 HCl A B CB CA 1. The Bronsted-Lowry theory of an acid-base reaction involves the transfer of protons or H ions between the acid and base. H 2 SO 4 - OH. A more general look at the theory is an acid as a proton donor and a base as a proton acceptor.
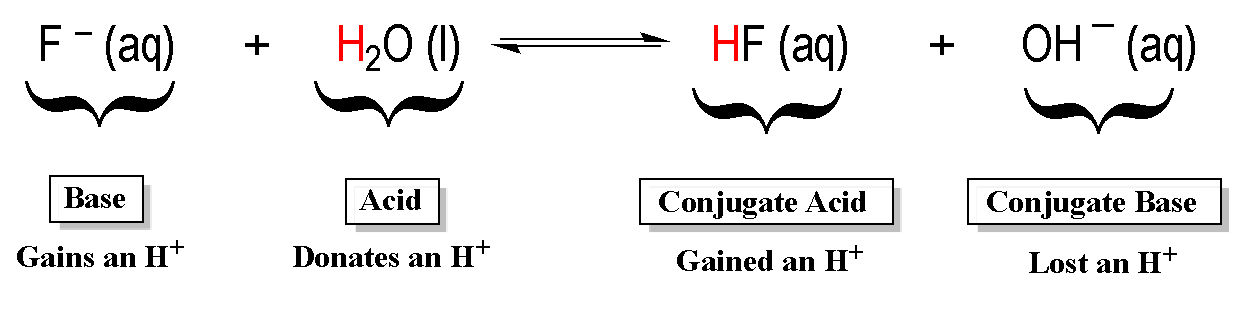 Source: clutchprep.com
Source: clutchprep.com
- H 2 O - Cl1 OH1 HCl A B CB CA 1. H 2 SO 4 - OH. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. An example of a proton acceptor is ammonia NH3. Identify the Bronsted-Lowry acids and bases using the definitions.
 Source: socratic.org
Source: socratic.org
Acid-base reactions dont have to occur in water however. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. When it donates its proton the acid becomes its conjugate baseA more general look at the theory is an acid as a proton donor and a base as a proton acceptor. What makes a solution an acid. Substance that donates a proton.
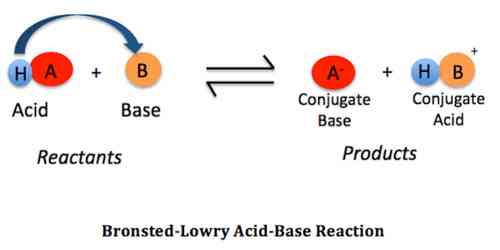 Source: qsstudy.com
Source: qsstudy.com
15 How do Brønsted bases differ from Lewis bases explain with example. According to Bronsted-Lowry theory an acid is a proton H1 donor and a base is a proton acceptor. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton. 10 What can act as a Bronsted-Lowry acid and base. A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction.
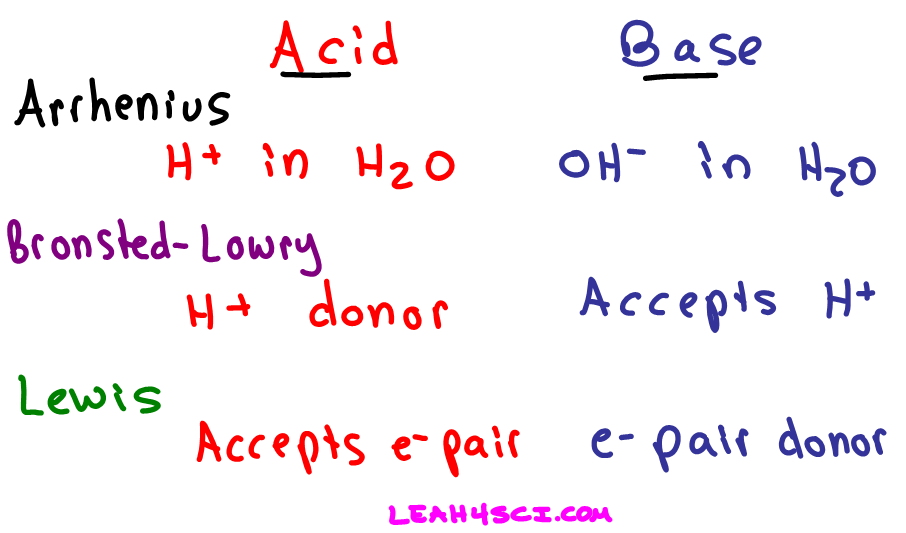 Source: leah4sci.com
Source: leah4sci.com
A Bronsted-Lowry base is a solution that is ready to accept protons in the form of hydrogen ions. In contrast a Bronsted-Lowry base accepts hydrogen ions. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. On the other hand water is the Bronsted-Lowry acid because it is the proton donor. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Bronsted-Lowry Acid Examples. The conjugate base is the hydroxide ion OH- because this is the substance produced when H2O donated the proton. In contrast to the acid definition a Bronsted-Lowry base is a substance that accepts protons. In contrast a Bronsted-Lowry base accepts hydrogen ions. - H 2 O - Cl1 OH1 HCl A B CB CA 1.
 Source: jove.com
Source: jove.com
A more general look at the theory is an acid as a proton donor and a base as a proton acceptor. Consider a reaction in which ammonia base is dissolved in water acid. Thus the products have conjugate acids and conjugate bases. The Bronsted-Lowry theory of an acid-base reaction involves the transfer of protons or H ions between the acid and base. Steps for Identifying Bronsted-Lowry Acids and Bases.
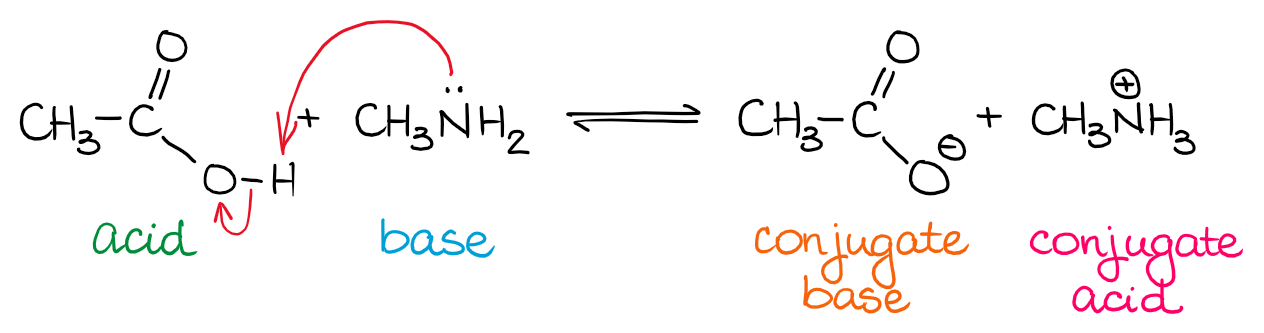 Source: organicchemistrytutor.com
Source: organicchemistrytutor.com
HCl NH_3 rightleftharpoons NH_4 Cl- Hydrochloric acid HCl is. A Bronsted-Lowry base is a solution that is ready to accept protons in the form of hydrogen ions. Bronsted - Lowry Base. Consider the reactions of ammonia with water and HCl. The ammonia is happy to accept a proton from the hydrogen of water H2O to become NH4NH3 H2O NH4 OH-.
 Source: slideplayer.com
Source: slideplayer.com
Learn the chemical characteristics of this type of acid and examples of several. Since a Bronsted Lowry acid cannot occur without the presence of a Bronsted Lowry base the two are linked in what is called conjugate pairs. A more general look at the theory is an acid as a proton donor and a base as a proton acceptor. Substance that donates a proton. Please refer to the example to see a Bronsted Lowry acid base pair and its conjugate pairs.
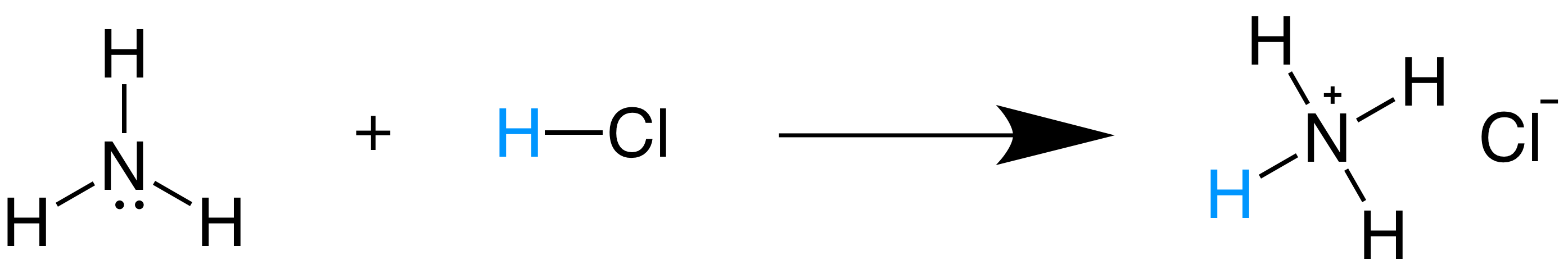 Source: khanacademy.org
Source: khanacademy.org
The Bronsted-Lowry acidbase definition requires a bit of analysis. Additionally because ammonia accepted the proton from hydrochloric acid the positive ammonia ion is the conjugate acid of the equation. The ammonia is happy to accept a proton from the hydrogen of water H2O to become NH4. According to the theory an acid and base react with each other causing the acid to form its conjugate base and the base to form its conjugate acid by exchanging a proton. Water has a limiting effect on the strength of acids and bases.
 Source: quizlet.com
Source: quizlet.com
On the other hand water is the Bronsted-Lowry acid because it is the proton donor. In contrast to the acid definition a Bronsted-Lowry base is a substance that accepts protons. There are many compounds that can accept a pair of electrons from a Lewis base yet dont have any Brønsted-Lowry acid proton moiety. The following Bronsted-Lowry bases list is arranged in order of decreasing base strength. On the other hand water is the Bronsted-Lowry acid because it is the proton donor.

An example of a proton acceptor is ammonia NH3. - H 2 O - Cl1 OH1 HCl A B CB CA 1. H 2 SO 4 - OH. A Bronsted-Lowry base is a solution that is ready to accept protons in the form of hydrogen ions. An example of a proton acceptor is ammonia NH3.
 Source: youtube.com
Source: youtube.com
What makes a solution an acid. 15 How do Brønsted bases differ from Lewis bases explain with example. Brønsted Acids and Bases in Nonaqueous Solutions. Vinegar lemon juice gastric juice soft drinks examples. Learn the chemical characteristics of this type of acid and examples of several.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title bronsted lowry acid examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






