Your Bent molecular geometry examples images are ready. Bent molecular geometry examples are a topic that is being searched for and liked by netizens today. You can Download the Bent molecular geometry examples files here. Get all royalty-free photos and vectors.
If you’re looking for bent molecular geometry examples images information connected with to the bent molecular geometry examples interest, you have come to the ideal blog. Our website frequently provides you with hints for seeking the maximum quality video and image content, please kindly hunt and find more informative video content and graphics that match your interests.
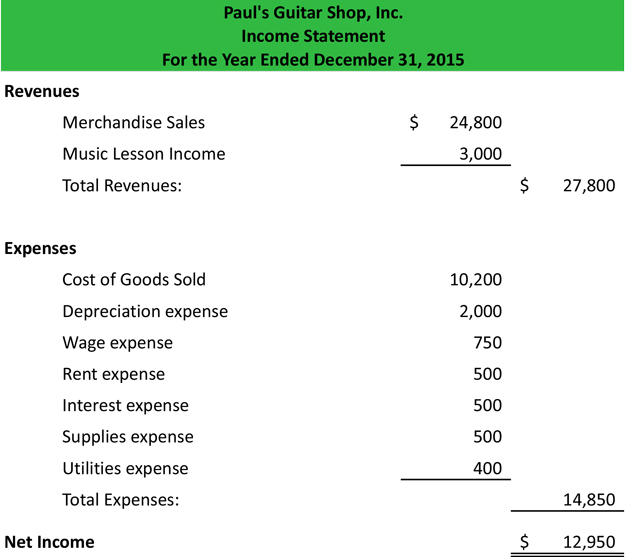
Bent Molecular Geometry Examples. The tetrahedral electron group geometry requires that the molecular geometry is bent. From 2 hydrogen atoms to complete its octet. The two atoms connected to the central atom form a molecule with a bent shape. Water H 2 O is an example of a bent molecule as well as its analogues.
 Water Molecules Are Polar In Nature Water Molecules Are Basically H 2o Molecules Which Have Bent Shapes Also O Atom Water Molecule Molecules Hydrogen Bond From pinterest.com
Water Molecules Are Polar In Nature Water Molecules Are Basically H 2o Molecules Which Have Bent Shapes Also O Atom Water Molecule Molecules Hydrogen Bond From pinterest.com
The two atoms connected to the central atom form a molecule with a bent shape. The shape of the orbitals is tetrahedral. According to Bents rule molecular geometry can be explained and predicted by changing the substituent group. Sulfur Dichloride is an angular molecule. Predicting molecular geometry. If there are two bond pairs and two lone pairs of electrons the molecular geometry is angular or bent eg.
B trigonal planar trigonal planar.
Number of Electron Domains Electronic Geometry Nonbonding Pairs Molecular Geometry Examples 2 Linear 0 AB 2 Linear CO 2 HCN 3 Trigonal Planar 0 AB 3 1 AB 2 E Trigonal Planar Bent BCl 3 O 3 SO 2 4 Tetrahedral 0 AB 4 1 AB 3 E 2 AB 2 E 2 Tetrahedral Trigonal Planar Bent CH 4 NH 3. Water H 2 O is an example of a bent molecule as well as its analogues. Molecules with three electron pairs have a domain geometry that is trigonal planar. The shape of the orbitals is tetrahedral. Furthermore why is a molecule bent. Table Summarizing Molecular Geometries.
 Source: pinterest.com
Source: pinterest.com
The water molecule is so common that it is wise to just memorize that water is a BENT molecule. Notice there are 4 attachments or electron groups surrounding oxygen. An example of trigonal pyramid molecular geometry that results from tetrahedral electron pair geometry is NH 3. This would make the electron geometry tetrahedral. Water H 2 O is an example of a bent molecule as well as its analogues.
 Source: pinterest.com
Source: pinterest.com
14 rows If we replace a bonding pair with a lone pair as in SO 2 the geometry is described as. Number of Electron Domains Electronic Geometry Nonbonding Pairs Molecular Geometry Examples 2 Linear 0 AB 2 Linear CO 2 HCN 3 Trigonal Planar 0 AB 3 1 AB 2 E Trigonal Planar Bent BCl 3 O 3 SO 2 4 Tetrahedral 0 AB 4 1 AB 3 E 2 AB 2 E 2 Tetrahedral Trigonal Planar Bent CH 4 NH 3. Molecular Geometry Chart. Definition Examples and Study Guides. This would make the electron geometry tetrahedral.
 Source: pinterest.com
Source: pinterest.com
However this is not the molecular geometry. Predicting molecular geometry. For bent molecular geometry when the electron-pair geometry is tetrahedral the bond angle is around 105 degrees. Number of Electron Domains Electronic Geometry Nonbonding Pairs Molecular Geometry Examples 2 Linear 0 AB 2 Linear CO 2 HCN 3 Trigonal Planar 0 AB 3 1 AB 2 E Trigonal Planar Bent BCl 3 O 3 SO 2 4 Tetrahedral 0 AB 4 1 AB 3 E 2 AB 2 E 2 Tetrahedral Trigonal Planar Bent CH 4 NH 3. 1045o H 2O has a dipole moment of 185D.
 Source: pinterest.com
Source: pinterest.com
The answer is the molecular geometry of water would be bent. Molecular Geometry Examples Sulfur Dichloride. Molecular Geometries Where Central Atom Has Lone Pairs Continued Original Shape without Lone Pairs of Outer Atoms of Lone Pairs General Formula Molecular Geometry Name trigonal planar AB3 2 1 AB2E bent or angular 3 1 AB3E trigonal pyramidal tetrahedral AB4 2 2 AB2E2 bent or angular. This model structure shows the 1095 o tetrahedral bond angles formed by a central sphere 4 smaller terminal spheres. With the highly electronegative halogen substituent Cl more p character is concentrated on central atom in.
 Source: pinterest.com
Source: pinterest.com
This would make the electron geometry tetrahedral. The oxygen has 6 valence electrons and thus needs 2 more electrons from 2 hydrogen atoms to complete its octet. 1045o H 2O has a dipole moment of 185D. The final 4 electron group geometry is the bent geometry. Sulfur Dichloride is an angular molecule.
 Source: pinterest.com
Source: pinterest.com
The oxygen has 6 valence electrons and thus needs 2 more electrons from 2. In the molecule Me 2 XCl 2 where Xmain group elements C Si Ge Sn Pb the bond angle ClXCl is smaller than the CXC bond angle. Water is an example of a. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal eg. The molecular geometry of NO 2 - is bent because the central nitrogen atom has 6 valence electrons negative charge on N four of which are used in forming two double bonds with oxygen atoms and the remaining two form a lone pair.
 Source: pinterest.com
Source: pinterest.com
In chemistry molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry also known as angular or V-shapedCertain atoms such as oxygen will almost always set their two or more covalent bonds in non-collinear directions due to their electron configuration. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. This model structure shows the 1095 o tetrahedral bond angles formed by a central sphere 4 smaller terminal spheres. The answer is the molecular geometry of water would be bent. 1045o H 2O has a dipole moment of 185D.
 Source: pinterest.com
Source: pinterest.com
This model structure shows the 1095 o tetrahedral bond angles formed by a central sphere 4 smaller terminal spheres. Solution continued As this example illustrates when a molecule exhibits resonance any one of the resonance structures can be used to predict the molecular. AJR Ch10 Molecular Geometrydocx Slide 12 In summary. Predict the electron-domain geometry and the molecular geometry for a SeCl. An example of trigonal pyramid molecular geometry that results from tetrahedral electron pair geometry is NH 3.
 Source: pinterest.com
Source: pinterest.com
Number of Electron Domains Electronic Geometry Nonbonding Pairs Molecular Geometry Examples 2 Linear 0 AB 2 Linear CO 2 HCN 3 Trigonal Planar 0 AB 3 1 AB 2 E Trigonal Planar Bent BCl 3 O 3 SO 2 4 Tetrahedral 0 AB 4 1 AB 3 E 2 AB 2 E 2 Tetrahedral Trigonal Planar Bent CH 4 NH 3. In other words lone pairs take up more space. Sulfur Dichloride is an angular molecule. The oxygen has 6 valence electrons and thus needs 2 more electrons from 2. This would make the electron geometry tetrahedral.
 Source: pinterest.com
Source: pinterest.com
Predict the electron-domain geometry and the molecular geometry for a SeCl. This subject uses geometric models to represent the shape and structure of molecules. The tetrahedral electron group geometry requires that the molecular geometry is bent. B trigonal planar trigonal planar. This then leaves a lone electron pair that is not bonded to any other atom.
 Source: pinterest.com
Source: pinterest.com
If there are two bond pairs and two lone pairs of electrons the molecular geometry is angular or bent eg. Molecular Geometries Where Central Atom Has Lone Pairs Continued Original Shape without Lone Pairs of Outer Atoms of Lone Pairs General Formula Molecular Geometry Name trigonal planar AB3 2 1 AB2E bent or angular 3 1 AB3E trigonal pyramidal tetrahedral AB4 2 2 AB2E2 bent or angular. This subject uses geometric models to represent the shape and structure of molecules. Here the lone pair on the central atom repels the electrons in the two bonds causing the atom to adopt a bent molecular geometry. Notice there are 4 attachments or electron groups surrounding oxygen.
 Source: pinterest.com
Source: pinterest.com
The molecular geometry of NO 2 - is bent because the central nitrogen atom has 6 valence electrons negative charge on N four of which are used in forming two double bonds with oxygen atoms and the remaining two form a lone pair. An example of bent molecular geometry that results from tetrahedral electron pair geometry is H 2 O. This results in a bent molecular shape. The molecular geometry of NO 2 - is bent because the central nitrogen atom has 6 valence electrons negative charge on N four of which are used in forming two double bonds with oxygen atoms and the remaining two form a lone pair. This occurs when there are 2 bonds and 2 lone pairs.
 Source: pinterest.com
Source: pinterest.com
Two of the orbitals contain lone pairs of electrons. Water is an example of a. The Relationship Between the Number of Places Where Valence Electrons Can Be Found and the Goemetry Around an Atom. The shape of the orbitals is tetrahedral. The molecular dipole moment bisects the H-O-H bond and is directed towards the oxygen.
 Source: pinterest.com
Source: pinterest.com
In chemistry molecules with a non-collinear arrangement of two adjacent bonds have bent molecular geometry also known as angular or V-shapedCertain atoms such as oxygen will almost always set their two or more covalent bonds in non-collinear directions due to their electron configuration. According to Bents rule molecular geometry can be explained and predicted by changing the substituent group. Predict the electron-domain geometry and the molecular geometry for a SeCl. Water is an example of a. The formula for methane is eqCH_4 eq.
 Source: pinterest.com
Source: pinterest.com
The water molecule is so common that it is wise to just memorize that water is a BENT molecule. Molecular geometry is tetrahedral eg. In the molecule Me 2 XCl 2 where Xmain group elements C Si Ge Sn Pb the bond angle ClXCl is smaller than the CXC bond angle. Molecular Geometry Chart. The water molecule is so common that it is wise to just memorize that water is a BENT molecule.
 Source: pinterest.com
Source: pinterest.com
1045o H 2O has a dipole moment of 185D. With the highly electronegative halogen substituent Cl more p character is concentrated on central atom in. Molecular geometry Examples 2 X 2 0 Linear CO2 N3-3 X 3 2 0 1 Trigonal planar Bent SO3 CO3 2- SO2 O3 NO2 - 4 X 4 3 2 0 1 2 Tetrahedral Trigonal pyramid Bent CH4 NH4 PO 4 3- H3O. An example of bent molecular geometry that results from tetrahedral electron pair geometry is H 2 O. This subject uses geometric models to represent the shape and structure of molecules.
 Source: pinterest.com
Source: pinterest.com
B trigonal planar trigonal planar. Two of the orbitals contain lone pairs of electrons. If there are two bond pairs and two lone pairs of electrons the molecular geometry is angular or bent eg. Predicting molecular geometry. This model structure shows the 1095 o tetrahedral bond angles formed by a central sphere 4 smaller terminal spheres.
 Source: pinterest.com
Source: pinterest.com
This occurs when there are 2 bonds and 2 lone pairs. Two of the orbitals contain lone pairs of electrons. The formula for methane is eqCH_4 eq. The water molecule is so common that it is wise to just memorize that water is a BENT molecule. According to Bents rule molecular geometry can be explained and predicted by changing the substituent group.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title bent molecular geometry examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.